Ionic, not the osmotic component, is responsible for the salinity-induced inhibition of greening in etiolated wheat (Triticum aestivum L. cv. Mv Béres) leaves: a comparative study
- PMID: 37861810
- PMCID: PMC10589150
- DOI: 10.1007/s00425-023-04255-4
Ionic, not the osmotic component, is responsible for the salinity-induced inhibition of greening in etiolated wheat (Triticum aestivum L. cv. Mv Béres) leaves: a comparative study
Abstract
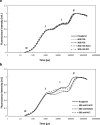
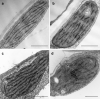
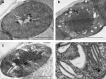
Greening was partially (in 300 mM NaCl, CaCl2, 600 mM KNO3 or KCl) or fully inhibited (in 600 mM NaCl, NaNO3 or NaCl:KCl) by the ionic and not the osmotic component of salinity. Although high soil salinity is an increasing global problem, not much is known about how direct exposure to salinity affects etiolated leaves of seedlings germinating in the soil and then reaching the surface. We investigated the effect of various salt treatments on the greening process of leaves in 8- to 11-day-old etiolated wheat (Triticum aestivum L. Mv. Béres) seedlings. Etiolated leaf segments pre-treated on different salt (600 mM NaCl:KCl 1:1, 600 mM NaCl, 600 mM KCl, 600 mM NaNO3, 600 mM KNO3, 300 mM KCl, 300 mM NaCl or 300 mM CaCl2) or isosmotic polyethylene glycol 6000 (PEG) solutions for 1.5 h in the dark and then greened for 16 h on the same solutions were studied. Leaf segments greened on PEG (osmotic stress) or on 300 mM KCl had similar chloroplasts compared to control samples greened on Hoagland solution. Slightly slower development of chloroplast structure and function (photosynthetic activity) was observed in segments greened on 300 mM NaCl or CaCl2, 600 mM KNO3 or KCl. However, etioplast-to-chloroplast transformation and chlorophyll accumulation were fully inhibited and peculiar prothylakoid swelling occurred in segments greened on 600 mM NaCl, NaNO3 or NaCl:KCl (1:1) solutions. The data indicate that not the high osmolarity of the used salt solution, but its ions, especially Na+, had the strongest negative impact on these processes.
Keywords: Chloroplast; Etioplast; Greening; Osmotic stress; Prolamellar body; Salt stress.
© 2023. The Author(s).
Conflict of interest statement
The authors have no competing interests to declare that are relevant to the content of this article.
Figures





References
-
- Abdelkader AF, Aronsson H, Sundqvist C. High salt stress in wheat leaves causes retardation of chlorophyll accumulation due to a limited rate of protochlorophyllide formation. Physiol Plant. 2007;130:157–166. doi: 10.1111/j.1399-3054.2007.00885.x. - DOI
MeSH terms
Substances
Grants and funding
- OTKA FK124748/Nemzeti Kutatási, Fejlesztési és Innovaciós Alap
- New National Excelence Programme (ÚNKP-22-5)/Nemzeti Kutatási, Fejlesztési és Innovaciós Alap
- Bolyai János Research Scholarship/Magyar Tudományos Akadémia
- Staff Mobility for Training Scholarship/Erasmus+
- Stipendium Hungaricum Scholarship/Tempus Közalapítvány
LinkOut - more resources
Full Text Sources

