Removal of Medicaid Prior Authorization Requirements and Buprenorphine Treatment for Opioid Use Disorder
- PMID: 37862034
- PMCID: PMC10589810
- DOI: 10.1001/jamahealthforum.2023.3549
Removal of Medicaid Prior Authorization Requirements and Buprenorphine Treatment for Opioid Use Disorder
Abstract
Importance: Buprenorphine treatment for opioid use disorder (OUD) is associated with decreased morbidity and mortality. Despite its effectiveness, buprenorphine uptake has been limited relative to the burden of OUD. Prior authorization (PA) policies may present a barrier to treatment, though research is limited, particularly in Medicaid populations.
Objective: To assess whether removal of Medicaid PAs for buprenorphine to treat OUD is associated with changes in buprenorphine prescriptions for Medicaid enrollees.
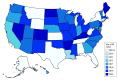
Design, setting, and participants: This state-level, serial cross-sectional study used quarterly data from 2015 through the first quarter (January-March) of 2019 to compare buprenorphine prescriptions in states that did and did not remove Medicaid PAs. Analyses were conducted between June 10, 2021, and August 15, 2023. The study included 23 states with active Medicaid PAs for buprenorphine in 2015 that required similar PA policies in fee-for-service and managed care plans and had at least 2 quarters of pre- and postperiod buprenorphine prescribing data.
Exposures: Removal of Medicaid PA for at least 1 formulation of buprenorphine for OUD.
Main outcomes and measures: The main outcome was number of quarterly buprenorphine prescriptions per 1000 Medicaid enrollees.
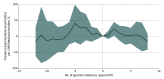
Results: Between 2015 and the first quarter of 2019, 6 states in the sample removed Medicaid PAs for at least 1 formulation of buprenorphine and had at least 2 quarters of pre- and postpolicy change data. Seventeen states maintained buprenorphine PAs throughout the study period. At baseline, relative to states that repealed PAs, states that maintained PAs had lower buprenorphine prescribing per 1000 Medicaid enrollees (median, 6.6 [IQR, 2.6-13.9] vs 24.1 [IQR, 8.7-27.5] prescriptions) and lower Medicaid managed care penetration (median, 38.5% [IQR, 0.0%-74.1%] vs 79.5% [IQR, 78.1%-83.5%] of enrollees) but similar opioid overdose rates and X-waivered buprenorphine clinicians per 100 000 population. In fully adjusted difference-in-differences models, removal of Medicaid PAs for buprenorphine was not associated with buprenorphine prescribing (1.4% decrease; 95% CI, -31.2% to 41.4%). For states with below-median baseline buprenorphine prescribing, PA removal was associated with increased buprenorphine prescriptions per 1000 Medicaid enrollees (40.1%; 95% CI, 0.6% to 95.1%), while states with above-median prescribing showed no change (-20.7%; 95% CI, -41.0% to 6.6%).
Conclusions and relevance: In this serial cross-sectional study of Medicaid PA policies for buprenorphine for OUD, removal of PAs was not associated with overall changes in buprenorphine prescribing among Medicaid enrollees. Given the ongoing burden of opioid overdoses, continued multipronged efforts are needed to remove barriers to buprenorphine care and increase availability of this lifesaving treatment.
Conflict of interest statement
Figures

References
-
- Priest KC, Gertner AK. State officials shouldn’t wait for federal action to increase opioid addiction treatment access. Health Affairs Blog, May 21, 2019. Accessed January 20, 2022. https://www.healthaffairs.org/content/forefront/state-officials-shouldn-...
-
- Beetham T. Buprenorphine prior authorization removal: low hanging fruit in the opioid overdose crisis. Harv Public Health Rev (Camb). 2019;25. doi: 10.54111/0001/Y2 - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

