Dried Blood Spots (DBS): A suitable alternative to using whole blood samples for diagnostic testing of visceral leishmaniasis in the post-elimination era
- PMID: 37862287
- PMCID: PMC10588855
- DOI: 10.1371/journal.pntd.0011680
Dried Blood Spots (DBS): A suitable alternative to using whole blood samples for diagnostic testing of visceral leishmaniasis in the post-elimination era
Abstract
Background: Serum or whole blood collection, processing, transport and storage still present significant challenges in low resource settings where mass surveillance is required to sustain disease elimination. Therefore, in this study, we explored the diagnostic efficacy of dried blood spots (DBS) as a minimally invasive and potentially cost-effective alternative sampling technique to whole blood sampling procedures for subsequent detection of Leishmania donovani antibodies or DNA.
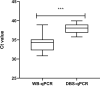
Methodology and principal findings: Archived serum, DNA samples from whole blood of visceral leishmaniasis (VL) cases and healthy controls, and DBS from corresponding cases and controls, were used. Both molecular and serological assays were optimized to detect L. donovani antibodies or DNA in DBS elute and results were compared against those obtained with whole blood. Serological assays (both rK28 ELISA and rK39 ELISA) of DBS samples showed sensitivity and specificity of 100% and had excellent agreement with results from whole blood samples (kappa value ranged from 0.98-1). Bland-Altman analysis of OD values from rK28-ELISA with DBS elute and patients' serum showed an excellent agreement (ICC = 0.9) whereas a good agreement (ICC = 0.8) was observed in the case of rK39-ELISA. However, qPCR and RPA of DBS samples had a diminished sensitivity of 76% and 68%, respectively, and poor agreement was observed with the whole blood samples.
Conclusion: Our results demonstrate that DBS offer excellent diagnostic efficiency for serological assays and represent a viable alternative to whole blood sampling procedures.
Copyright: © 2023 Ghosh et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures






Similar articles
-
Evaluation of Loopamp™ Leishmania Detection Kit and Leishmania Antigen ELISA for Post-Elimination Detection and Management of Visceral Leishmaniasis in Bangladesh.Front Cell Infect Microbiol. 2021 Apr 26;11:670759. doi: 10.3389/fcimb.2021.670759. eCollection 2021. Front Cell Infect Microbiol. 2021. PMID: 33981632 Free PMC article.
-
Evaluation of diagnostic performance of rK28 ELISA using urine for diagnosis of visceral leishmaniasis.Parasit Vectors. 2016 Jul 4;9(1):383. doi: 10.1186/s13071-016-1667-2. Parasit Vectors. 2016. PMID: 27377266 Free PMC article.
-
Performance of rK39-based immunochromatographic rapid diagnostic test for serodiagnosis of visceral leishmaniasis using whole blood, serum and oral fluid.PLoS One. 2020 Apr 2;15(4):e0230610. doi: 10.1371/journal.pone.0230610. eCollection 2020. PLoS One. 2020. PMID: 32240188 Free PMC article.
-
Use of dried blood spots in the detection of coronavirus disease 2019 (COVID-19): A systematic review.Indian J Med Microbiol. 2024 Sep-Oct;51:100700. doi: 10.1016/j.ijmmb.2024.100700. Epub 2024 Aug 13. Indian J Med Microbiol. 2024. PMID: 39127256
-
Reliability of dried blood spot (DBS) cards in antibody measurement: A systematic review.PLoS One. 2021 Mar 15;16(3):e0248218. doi: 10.1371/journal.pone.0248218. eCollection 2021. PLoS One. 2021. PMID: 33720928 Free PMC article.
Cited by
-
The Use of Dried Matrix Spots as an Alternative Sampling Technique for Monitoring Neglected Tropical Diseases.Pathogens. 2024 Aug 29;13(9):734. doi: 10.3390/pathogens13090734. Pathogens. 2024. PMID: 39338925 Free PMC article. Review.
References
-
- Hossain F, Ghosh P, Khan MAA, Duthie MS, Vallur AC, Picone A, et al. Real-time PCR in detection and quantitation of Leishmania donovani for the diagnosis of Visceral Leishmaniasis patients and the monitoring of their response to treatment. PLoS One. 2017;12(9):e0185606. doi: 10.1371/journal.pone.0185606 - DOI - PMC - PubMed
-
- Moretti M, Manfredi A, Freni F, Previderé C, Osculati AMM, Grignani P, et al. A comparison between two different dried blood substrates in determination of psychoactive substances in postmortem samples. Forensic Toxicol [Internet]. 2021;39(2):385–93. Available from: doi: 10.1007/s11419-020-00567-2 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

