Hunting for the elusive target antigen in gestational alloimmune liver disease (GALD)
- PMID: 37862305
- PMCID: PMC10588877
- DOI: 10.1371/journal.pone.0286432
Hunting for the elusive target antigen in gestational alloimmune liver disease (GALD)
Abstract
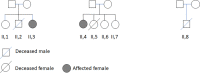
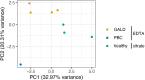
The prevailing concept is that gestational alloimmune liver disease (GALD) is caused by maternal antibodies targeting a currently unknown antigen on the liver of the fetus. This leads to deposition of complement on the fetal hepatocytes and death of the fetal hepatocytes and extensive liver injury. In many cases, the newborn dies. In subsequent pregnancies early treatment of the woman with intravenous immunoglobulin can be instituted, and the prognosis for the fetus will be excellent. Without treatment the prognosis can be severe. Crucial improvements of diagnosis require identification of the target antigen. For this identification, this work was based on two hypotheses: 1. The GALD antigen is exclusively expressed in the fetal liver during normal fetal life in all pregnancies; 2. The GALD antigen is an alloantigen expressed in the fetal liver with the woman being homozygous for the minor allele and the father being, most frequently, homozygous for the major allele. We used three different experimental approaches to identify the liver target antigen of maternal antibodies from women who had given birth to a baby with the clinical GALD diagnosis: 1. Immunoprecipitation of antigens from either a human liver cell line or human fetal livers by immunoprecipitation with maternal antibodies followed by mass spectrometry analysis of captured antigens; 2. Construction of a cDNA expression library from human fetal liver mRNA and screening about 1.3 million recombinants in Escherichia coli using antibodies from mothers of babies diagnosed with GALD; 3. Exome/genome sequencing of DNA from 26 presumably unrelated women who had previously given birth to a child with GALD with husband controls and supplementary HLA typing. In conclusion, using the three experimental approaches we did not identify the GALD target antigen and the exome/genome sequencing results did not support the hypothesis that the GALD antigen is an alloantigen, but the results do not yield basis for excluding that the antigen is exclusively expressed during fetal life., which is the hypothesis we favor.
Copyright: © 2023 Rieneck et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures





References
-
- Erlichman J, Loomes KM. https://www.uptodate.com/contents/causes-of-cholestasis-in-neonates-and-... 2021 [updated Mar 2021].
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

