Surfaceome analysis of extracellular vesicles from senescent cells uncovers uptake repressor DPP4
- PMID: 37862381
- PMCID: PMC10614838
- DOI: 10.1073/pnas.2219801120
Surfaceome analysis of extracellular vesicles from senescent cells uncovers uptake repressor DPP4
Abstract
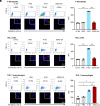
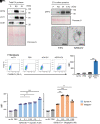
Senescent cells are beneficial for repairing acute tissue damage, but they are harmful when they accumulate in tissues, as occurs with advancing age. Senescence-associated extracellular vesicles (S-EVs) can mediate cell-to-cell communication and export intracellular content to the microenvironment of aging tissues. Here, we studied the uptake of EVs from senescent cells (S-EVs) and proliferating cells (P-EVs) and found that P-EVs were readily taken up by proliferating cells (fibroblasts and cervical cancer cells) while S-EVs were not. We thus investigated the surface proteome (surfaceome) of P-EVs relative to S-EVs derived from cells that had reached senescence via replicative exhaustion, exposure to ionizing radiation, or treatment with etoposide. We found that relative to P-EVs, S-EVs from all senescence models were enriched in proteins DPP4, ANXA1, ANXA6, S10AB, AT1A1, and EPHB2. Among them, DPP4 was found to selectively prevent uptake by proliferating cells, as ectopic overexpression of DPP4 in HeLa cells rendered DPP4-expressing EVs that were no longer taken up by other proliferating cells. We propose that DPP4 on the surface of S-EVs makes these EVs refractory to internalization by proliferating cells, advancing our knowledge of the impact of senescent cells in aging-associated processes.
Keywords: extracellular vesicles; senescence; surfaceome.
Conflict of interest statement
The authors declare no competing interest.
Figures




References
-
- Kovacic J. C., Moreno P., Nabel E. G., Hachinski V., Fuster V., Cellular senescence, vascular disease, and aging: Part 2 of a 2-part review: Clinical vascular disease in the elderly. Circulation 123, 1900–1910 (2011). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

