Identification of a carbonic anhydrase-Rubisco complex within the alpha-carboxysome
- PMID: 37862384
- PMCID: PMC10614612
- DOI: 10.1073/pnas.2308600120
Identification of a carbonic anhydrase-Rubisco complex within the alpha-carboxysome
Abstract
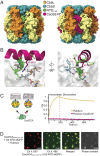
Carboxysomes are proteinaceous organelles that encapsulate key enzymes of CO2 fixation-Rubisco and carbonic anhydrase-and are the centerpiece of the bacterial CO2 concentrating mechanism (CCM). In the CCM, actively accumulated cytosolic bicarbonate diffuses into the carboxysome and is converted to CO2 by carbonic anhydrase, producing a high CO2 concentration near Rubisco and ensuring efficient carboxylation. Self-assembly of the α-carboxysome is orchestrated by the intrinsically disordered scaffolding protein, CsoS2, which interacts with both Rubisco and carboxysomal shell proteins, but it is unknown how the carbonic anhydrase, CsoSCA, is incorporated into the α-carboxysome. Here, we present the structural basis of carbonic anhydrase encapsulation into α-carboxysomes from Halothiobacillus neapolitanus. We find that CsoSCA interacts directly with Rubisco via an intrinsically disordered N-terminal domain. A 1.98 Å single-particle cryoelectron microscopy structure of Rubisco in complex with this peptide reveals that CsoSCA binding is predominantly mediated by a network of hydrogen bonds. CsoSCA's binding site overlaps with that of CsoS2, but the two proteins utilize substantially different motifs and modes of binding, revealing a plasticity of the Rubisco binding site. Our results advance the understanding of carboxysome biogenesis and highlight the importance of Rubisco, not only as an enzyme but also as a central hub for mediating assembly through protein interactions.
Keywords: CO2 fixation; carbonic anhydrase; carboxysome; cryoelectron microscopy; protein–protein interactions.
Conflict of interest statement
The authors declare no competing interest.
Figures




Comment in
-
The ties that bind. Disordered linkers underpin carboxysome construction.Proc Natl Acad Sci U S A. 2023 Nov 7;120(45):e2316828120. doi: 10.1073/pnas.2316828120. Epub 2023 Oct 27. Proc Natl Acad Sci U S A. 2023. PMID: 37889932 Free PMC article. No abstract available.
References
-
- Supuran C. T., Carbonic anhydrases: Novel therapeutic applications for inhibitors and activators. Nat. Rev. Drug Discov. 7, 168–181 (2008). - PubMed
-
- Badger M. R., Price G. D., The role of carbonic anhydrase in photosynthesis. Annu. Rev. Plant Physiol. Plant Mol. Biol. 45, 369–392 (1994).
-
- Andersson I., Catalysis and regulation in Rubisco. J. Exp. Bot. 59, 1555–1568 (2008). - PubMed

