Allosteric TYK2 inhibition: redefining autoimmune disease therapy beyond JAK1-3 inhibitors
- PMID: 37863021
- PMCID: PMC10589750
- DOI: 10.1016/j.ebiom.2023.104840
Allosteric TYK2 inhibition: redefining autoimmune disease therapy beyond JAK1-3 inhibitors
Abstract
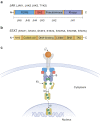
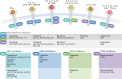
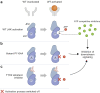
JAK inhibitors impact multiple cytokine pathways simultaneously, enabling high efficacy in treating complex diseases such as cancers and immune-mediated disorders. However, their broad reach also poses safety concerns, which have fuelled a demand for increasingly selective JAK inhibitors. Deucravacitinib, a first-in-class allosteric TYK2 inhibitor, represents a remarkable advancement in the field. Rather than competing at kinase domain catalytic sites as classical JAK1-3 inhibitors, deucravacitinib targets the regulatory pseudokinase domain of TYK2. It strikingly mirrors the functional effect of an evolutionary conserved naturally occurring TYK2 variant, P1104A, known to protect against multiple autoimmune diseases yet provide sufficient TYK2-mediated cytokine signalling required to prevent immune deficiency. The unprecedentedly high functional selectivity and efficacy-safety profile of deucravacitinib, initially demonstrated in psoriasis, combined with genetic support, and promising outcomes in early SLE clinical trials make this inhibitor ripe for exploration in other autoimmune diseases for which better, safe, and efficacious treatments are urgently needed.
Keywords: Autoimmune disease; Deucravacitinib; JAK inhibitors; TYK2.
Copyright © 2023 The Author(s). Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of interests L.F. and K.E.A. have an unrestricted grant from Bristol Myers Squibb. L.T.J. and M.F. declare no competing interests.
Figures

References
-
- Zhou Y.J., Chen M., Cusack N.A., et al. Unexpected effects of FERM domain mutations on catalytic activity of Jak3: structural implication for Janus kinases. Mol Cell. 2001;8(5):959–969. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

