The multi-lineage transcription factor ISL1 controls cardiomyocyte cell fate through interaction with NKX2.5
- PMID: 37863045
- PMCID: PMC10679653
- DOI: 10.1016/j.stemcr.2023.09.014
The multi-lineage transcription factor ISL1 controls cardiomyocyte cell fate through interaction with NKX2.5
Abstract
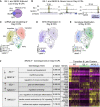
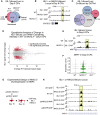
Congenital heart disease often arises from perturbations of transcription factors (TFs) that guide cardiac development. ISLET1 (ISL1) is a TF that influences early cardiac cell fate, as well as differentiation of other cell types including motor neuron progenitors (MNPs) and pancreatic islet cells. While lineage specificity of ISL1 function is likely achieved through combinatorial interactions, its essential cardiac interacting partners are unknown. By assaying ISL1 genomic occupancy in human induced pluripotent stem cell-derived cardiac progenitors (CPs) or MNPs and leveraging the deep learning approach BPNet, we identified motifs of other TFs that predicted ISL1 occupancy in each lineage, with NKX2.5 and GATA motifs being most closely associated to ISL1 in CPs. Experimentally, nearly two-thirds of ISL1-bound loci were co-occupied by NKX2.5 and/or GATA4. Removal of NKX2.5 from CPs led to widespread ISL1 redistribution, and overexpression of NKX2.5 in MNPs led to ISL1 occupancy of CP-specific loci. These results reveal how ISL1 guides lineage choices through a combinatorial code that dictates genomic occupancy and transcription.
Keywords: ISL1; NKX2.5; cardiac development; cardiac progenitor; cell specification; combinatorial code; transcription factor motifs; transcription factors; transcriptional regulation.
Copyright © 2023 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests D.S. is a co-founder and member of the board of directors of Tenaya Therapeutics and has equity in Tenaya Therapeutics. K.N.I. is an employee and shareholder of Tenaya Therapeutics. N.J.K. has received research support from Vir Biotechnology, F. Hoffmann-La Roche, and Rezo Therapeutics. N.J.K. has financially compensated consulting agreements with Maze Therapeutics, Interline Therapeutics, Rezo Therapeutics, and GEn1E Lifesciences, Inc. He is on the Board of Directors of Rezo Therapeutics and is a shareholder in Tenaya Therapeutics, Maze Therapeutics, Rezo Therapeutics, and Interline Therapeutics.
Figures




References
-
- Anderson D.J., Kaplan D.I., Bell K.M., Koutsis K., Haynes J.M., Mills R.J., Phelan D.G., Qian E.L., Leitoguinho A.R., Arasaratnam D., et al. NKX2-5 regulates human cardiomyogenesis via a HEY2 dependent transcriptional network. Nat. Commun. 2018;9:1373. doi: 10.1038/s41467-018-03714-x. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

