Evolutionary Insights from a Large-Scale Survey of Population-Genomic Variation
- PMID: 37863047
- PMCID: PMC10630549
- DOI: 10.1093/molbev/msad233
Evolutionary Insights from a Large-Scale Survey of Population-Genomic Variation
Abstract
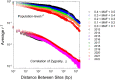
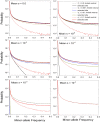
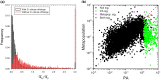
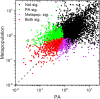
The field of genomics has ushered in new methods for studying molecular-genetic variation in natural populations. However, most population-genomic studies still rely on small sample sizes (typically, <100 individuals) from single time points, leaving considerable uncertainties with respect to the behavior of relatively young (and rare) alleles and, owing to the large sampling variance of measures of variation, to the specific gene targets of unusually strong selection. Genomic sequences of ∼1,700 haplotypes distributed over a 10-year period from a natural population of the microcrustacean Daphnia pulex reveal evolutionary-genomic features at a refined scale, including previously hidden information on the behavior of rare alleles predicted by recent theory. Background selection, resulting from the recurrent introduction of deleterious alleles, appears to strongly influence the dynamics of neutral alleles, inducing indirect negative selection on rare variants and positive selection on common variants. Temporally fluctuating selection increases the persistence of nonsynonymous alleles with intermediate frequencies, while reducing standing levels of variation at linked silent sites. Combined with the results from an equally large metapopulation survey of the study species, classes of genes that are under strong positive selection can now be confidently identified in this key model organism. Most notable among rapidly evolving Daphnia genes are those associated with ribosomes, mitochondrial functions, sensory systems, and lifespan determination.
Keywords: Daphnia pulex; adaptive divergence; background selection; fluctuating selection; linkage disequilibrium; population genomics; site-frequency spectrum.
© The Author(s) 2023. Published by Oxford University Press on behalf of Society for Molecular Biology and Evolution.
Conflict of interest statement
Conflict of interest statement. None declared.
Figures



Update of
-
Evolutionary Insights from a Large-scale Survey of Population-genomic Variation.bioRxiv [Preprint]. 2023 May 3:2023.05.03.539276. doi: 10.1101/2023.05.03.539276. bioRxiv. 2023. Update in: Mol Biol Evol. 2023 Nov 3;40(11):msad233. doi: 10.1093/molbev/msad233. PMID: 37205430 Free PMC article. Updated. Preprint.
References
Publication types
MeSH terms
Associated data
- SRA/PRJNA684968
- SRA/SAMN12816670
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources

