Shiga toxin targets the podocyte causing hemolytic uremic syndrome through endothelial complement activation
- PMID: 37863058
- PMCID: PMC7617617
- DOI: 10.1016/j.medj.2023.09.002
Shiga toxin targets the podocyte causing hemolytic uremic syndrome through endothelial complement activation
Abstract
Background: Shiga toxin (Stx)-producing Escherichia coli hemolytic uremic syndrome (STEC-HUS) is the leading cause of acute kidney injury in children, with an associated mortality of up to 5%. The mechanisms underlying STEC-HUS and why the glomerular microvasculature is so susceptible to injury following systemic Stx infection are unclear.
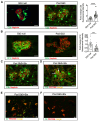
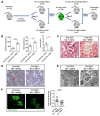
Methods: Transgenic mice were engineered to express the Stx receptor (Gb3) exclusively in their kidney podocytes (Pod-Gb3) and challenged with systemic Stx. Human glomerular cell models and kidney biopsies from patients with STEC-HUS were also studied.
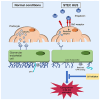
Findings: Stx-challenged Pod-Gb3 mice developed STEC-HUS. This was mediated by a reduction in podocyte vascular endothelial growth factor A (VEGF-A), which led to loss of glomerular endothelial cell (GEnC) glycocalyx, a reduction in GEnC inhibitory complement factor H binding, and local activation of the complement pathway. Early therapeutic inhibition of the terminal complement pathway with a C5 inhibitor rescued this podocyte-driven, Stx-induced HUS phenotype.
Conclusions: This study potentially explains why systemic Stx exposure targets the glomerulus and supports the early use of terminal complement pathway inhibition in this devastating disease.
Funding: This work was supported by the UK Medical Research Council (MRC) (grant nos. G0901987 and MR/K010492/1) and Kidney Research UK (grant nos. TF_007_20151127, RP42/2012, and SP/FSGS1/2013). The Mary Lyon Center is part of the MRC Harwell Institute and is funded by the MRC (A410).
Keywords: Pre-clinical research; pre-clinical research.
Copyright © 2023 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures




References
-
- Garg AX, Suri RS, Barrowman N, Rehman F, Matsell D, Rosas-Arellano MP, Salvadori M, Haynes RB, Clark WF. Long-term renal prognosis of diarrhea-associated hemolytic uremic syndrome: a systematic review, meta-analysis, and meta-regression. JAMA. 2003;290:1360–1370. - PubMed
-
- George JN, Nester CM. Syndromes of thrombotic microangiopathy. N Engl J Med. 2014;371:654–666. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

