Thermodynamic model of the oxidation of Ln-doped UO2
- PMID: 37863981
- PMCID: PMC10589314
- DOI: 10.1038/s41598-023-42616-x
Thermodynamic model of the oxidation of Ln-doped UO2
Abstract
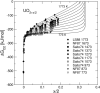
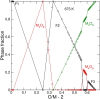
Ln-doped UO2 is often considered as a model system of spent nuclear fuel (SNF) helping to reveal effects of fission and activation products on its chemical stability. Comparing thermodynamics of UO2-UO3 and LnO1.5-UO2-UO3 systems provides a means to understand the phenomenon of an increased resistivity of Ln-doped UO2 to oxidation in air relative to pure UO2. Here a thermodynamic model is developed and is applied to investigate detailed phase changes occurring along the oxidation of Ln-doped fluorite to U3O8. The study proposes that an enhanced resistivity to oxidation of Ln-doped UO2 is likely caused by a thermodynamically driven partitioning of Ln between a fluorite-type phase and a U3O8 polymorph, which at ambient temperatures becomes hindered by slow diffusion.
© 2023. Springer Nature Limited.
Conflict of interest statement
The authors declare no competing interests.
Figures





References
-
- Kleykamp H. The chemical state of fission products in oxide fuels at different stages of the nuclear fuel cycle. Nucl. Tech. 1988;80:412–422. doi: 10.13182/NT88-A34065. - DOI
-
- Carbol, P., Wegen, D. H., Wiss, T. & Fors, P. Spent nuclear fuel as waste material in Comprehensive Nuclear Materials (ed. Konings, R.) 389–420 (Elsevier, 2012).
-
- Böhm W, Kiehlmann HD, Neufert A, Peehs M. Gd2O3 up to 9 weight percent, an established burnable poison for advanced fuel management in pressurized water reactors. Kerntechnik. 1987;50:234–240. doi: 10.1515/kern-1987-500411. - DOI
-
- Kim J-S, Kwon S-K, Sanchez M, Cho G-C. Geological storage of high-level nuclear waste. KSCE J. Civ. Eng. 2011;15:721–737. doi: 10.1007/s12205-011-0012-8. - DOI
-
- Kertes AS, Guillaumont R. Solubility of UO2. A comparative review. Nucl. Chem. Waste Manag. 1985;5:215–219. doi: 10.1016/0191-815X(85)90080-4. - DOI
LinkOut - more resources
Full Text Sources

