National trends in neoadjuvant chemotherapy utilization in patients with early-stage node-negative triple-negative breast cancer: the impact of the CREATE-X trial
- PMID: 37864105
- PMCID: PMC10872271
- DOI: 10.1007/s10549-023-07114-8
National trends in neoadjuvant chemotherapy utilization in patients with early-stage node-negative triple-negative breast cancer: the impact of the CREATE-X trial
Abstract
Purpose: Neoadjuvant chemotherapy (NAC) for triple-negative breast cancer (TNBC) allows for assessment of tumor pathological response and has survival implications. In 2017, the CREATE-X trial demonstrated survival benefit with adjuvant capecitabine in patients TNBC and residual disease after NAC. We aimed to assess national rates of NAC for cT1-2N0M0 TNBC before and after CREATE-X and examine factors associated with receiving NAC vs adjuvant chemotherapy (AC).
Methods: A retrospective cohort study of women with cT1-2N0M0 TNBC diagnosed from 2014 to 2019 in the National Cancer Database (NCDB) was performed. Variables were analyzed via ANOVA, Chi-squared, Fisher Exact tests, and a multivariate linear regression model was created.
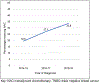
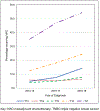
Results: 55,633 women were included: 26.9% received NAC, 52.4% AC, and 20.7% received no chemotherapy (median ages 53, 59, and 71 years, p < 0.01). NAC utilization significantly increased over time: 19.5% in 2014-15 (n = 3,465 of 17,777), 27.1% in 2016-17 (n = 5,140 of 18,985), and 33.6% in 2018-19 (n = 6,337 of 18,871, p < 0.001). On multivariate analysis, increased NAC was associated with younger age (< 50), non-Hispanic white race/ethnicity, lack of comorbidities, cT2 tumors, care at an academic or integrated-network cancer program, and diagnosis post-2017 (p < 0.05 for all). Patients with government-provided insurance were less likely to receive NAC (p < 0.01). Women who traveled > 60 miles for treatment were more likely to receive NAC (p < 0.01).
Conclusion: From 2014 to 2019, NAC utilization increased for patients with cT1-2N0M0 TNBC. Racial, socioeconomic, and access disparities were observed in who received NAC vs AC and warrants interventions to ensure equitable care.
Keywords: Breast cancer; CREATEX; Cancer disparities; Disparities; Early-stage breast cancer; Healthcare access; Neoadjuvant chemotherapy; Triple-negative breast cancer.
© 2023. The Author(s), under exclusive licence to Springer Science+Business Media, LLC, part of Springer Nature.
Conflict of interest statement
Figures

Similar articles
-
Association of Low Nodal Positivity Rate Among Patients With ERBB2-Positive or Triple-Negative Breast Cancer and Breast Pathologic Complete Response to Neoadjuvant Chemotherapy.JAMA Surg. 2018 Dec 1;153(12):1120-1126. doi: 10.1001/jamasurg.2018.2696. JAMA Surg. 2018. PMID: 30193375 Free PMC article.
-
Does Neoadjuvant Chemotherapy in Clinical T1-T2 N0 Triple-Negative Breast Cancer Increase the Extent of Axillary Surgery?Ann Surg Oncol. 2024 May;31(5):3128-3140. doi: 10.1245/s10434-024-14914-9. Epub 2024 Jan 25. Ann Surg Oncol. 2024. PMID: 38270828 Free PMC article.
-
Clinical outcomes with neoadjuvant versus adjuvant chemotherapy for triple negative breast cancer: A report from the National Cancer Database.PLoS One. 2019 Sep 19;14(9):e0222358. doi: 10.1371/journal.pone.0222358. eCollection 2019. PLoS One. 2019. PMID: 31536530 Free PMC article.
-
Capecitabine for hormone receptor-positive versus hormone receptor-negative breast cancer.Cochrane Database Syst Rev. 2021 May 26;5(5):CD011220. doi: 10.1002/14651858.CD011220.pub2. Cochrane Database Syst Rev. 2021. PMID: 34037241 Free PMC article.
-
The role of capecitabine-based neoadjuvant and adjuvant chemotherapy in early-stage triple-negative breast cancer: a systematic review and meta-analysis.BMC Cancer. 2021 Jan 19;21(1):78. doi: 10.1186/s12885-021-07791-y. BMC Cancer. 2021. PMID: 33468087 Free PMC article.
Cited by
-
ASO Author Reflections: Neoadjuvant Systemic Therapy and Nodal Management in cT1-2 N0 Triple-Negative Breast Cancer.Ann Surg Oncol. 2024 May;31(5):3196-3197. doi: 10.1245/s10434-024-15020-6. Epub 2024 Feb 10. Ann Surg Oncol. 2024. PMID: 38341380 Free PMC article. No abstract available.
-
Does concomitant ductal carcinoma in situ influence the prognostic outcome after neoadjuvant therapy in triple-negative invasive ductal carcinoma?World J Surg Oncol. 2025 Mar 25;23(1):101. doi: 10.1186/s12957-025-03753-x. World J Surg Oncol. 2025. PMID: 40133928 Free PMC article.
-
The Prevalence of Sentinel Lymph Node Positivity and Implications for the Utility of Frozen Section Diagnosis Following Neoadjuvant Systemic Therapy in Patients with Clinically Node-Negative HER2-Positive or Triple-Negative Breast Cancer.Ann Surg Oncol. 2024 Oct;31(11):7339-7346. doi: 10.1245/s10434-024-15712-z. Epub 2024 Jul 24. Ann Surg Oncol. 2024. PMID: 39048903
-
National trends in neoadjuvant systemic therapy utilization in patients with early-stage HER2-positive breast cancer from 2016-2021: The impact of the KATHERINE trial.Breast Cancer Res Treat. 2025 Aug;212(3):531-543. doi: 10.1007/s10549-025-07750-2. Epub 2025 Jun 3. Breast Cancer Res Treat. 2025. PMID: 40459676
-
Clinical outcomes of early-stage triple-negative breast cancer after neoadjuvant chemotherapy according to HER2-low status☆.ESMO Open. 2024 Nov;9(11):103973. doi: 10.1016/j.esmoop.2024.103973. Epub 2024 Nov 4. ESMO Open. 2024. PMID: 39500139 Free PMC article.
References
-
- Mittendorf EA, Zhang H, Barrios CH et al. (2020) Neoadjuvant atezolizumab in combination with sequential nab-paclitaxel and anthracycline-based chemotherapy versus placebo and chemotherapy in patients with early-stage triple-negative breast cancer (IMpassion031): a randomised, double-blind, phase 3 trial. Lancet 396(10257):1090–1100. 10.1016/S0140-6736(20)31953-X - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

