Efficacy and safety of mesenchymal stem cell therapy in liver cirrhosis: a systematic review and meta-analysis
- PMID: 37864199
- PMCID: PMC10590028
- DOI: 10.1186/s13287-023-03518-x
Efficacy and safety of mesenchymal stem cell therapy in liver cirrhosis: a systematic review and meta-analysis
Abstract
Aim: Although the efficacy and safety of mesenchymal stem cell therapy for liver cirrhosis have been demonstrated in several studies. Clinical cases of mesenchymal stem cell therapy for patients with liver cirrhosis are limited and these studies lack the consistency of treatment effects. This article aimed to systematically investigate the efficacy and safety of mesenchymal stem cells in the treatment of liver cirrhosis.
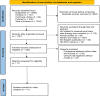
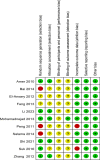
Method: The data source included PubMed/Medline, Web of Science, EMBASE, and Cochrane Library, from inception to May 2023. Literature was screened by the PICOS principle, followed by literature quality evaluation to assess the risk of bias. Finally, the data from each study's outcome indicators were extracted for a combined analysis. Outcome indicators of the assessment included liver functions and adverse events. Statistical analysis was performed using Review Manager 5.4.
Results: A total of 11 clinical trials met the selection criteria. The pooled analysis' findings demonstrated that both primary and secondary indicators had improved. Compared to the control group, infusion of mesenchymal stem cells significantly increased ALB levels in 2 weeks, 1 month, 3 months, and 6 months, and significantly decreased MELD score in 1 month, 2 months, and 6 months, according to a subgroup analysis using a random-effects model. Additionally, the hepatic arterial injection favored improvements in MELD score and ALB levels. Importantly, none of the included studies indicated any severe adverse effects.
Conclusion: The results showed that mesenchymal stem cell was effective and safe in the treatment of liver cirrhosis, improving liver function (such as a decrease in MELD score and an increase in ALB levels) in patients with liver cirrhosis and exerting protective effects on complications of liver cirrhosis and the incidence of hepatocellular carcinoma. Although the results of the subgroup analysis were informative for the selection of mesenchymal stem cells for clinical treatment, a large number of high-quality randomized controlled trials validations are still needed.
Keywords: Efficacy; Liver cirrhosis; Mesenchymal stem cells; Meta-analysis; Safety.
© 2023. BioMed Central Ltd., part of Springer Nature.
Conflict of interest statement
The authors claim that they have no conflict of interest.
Figures

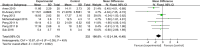








References
-
- Asrani SK, Devarbhavi H, Eaton J, Kamath PS. Burden of liver diseases in the world. J Hepatol. 2019;70(1):151–171. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

