Impact of clinical decision support on controlled substance prescribing
- PMID: 37864226
- PMCID: PMC10588193
- DOI: 10.1186/s12911-023-02314-0
Impact of clinical decision support on controlled substance prescribing
Abstract
Background: Prescription drug overdose and misuse has reached alarming numbers. A persistent problem in clinical care is lack of easy, immediate access to all relevant information at the actionable time. Prescribers must digest an overwhelming amount of information from each patient's record as well as remain up-to-date with current evidence to provide optimal care. This study aimed to describe prescriber response to a prospective clinical decision support intervention designed to identify patients at risk of adverse events associated with misuse of prescription opioids/benzodiazepines and promote adherence to clinical practice guidelines.
Methods: This study was conducted at a large multi-center healthcare system, using data from the electronic health record. A prospective observational study was performed as clinical decision support (CDS) interventions were sequentially launched (January 2016-July 2019). All data were captured from the medical record prospectively via the CDS tools implemented. A consecutive series of all patient encounters including an opioid/benzodiazepine prescription were included in this study (n = 61,124,172 encounters; n = 674,785 patients). Physician response to the CDS interventions was the primary outcome, and it was assessed over time using control charts.
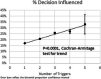
Results: An alert was triggered in 23.5% of encounters with a prescription (n = 555,626). The prescriber decision was influenced in 18.1% of these encounters (n = 100,301). As the number of risk factors increased, the rate of decision being influenced also increased (p = 0.0001). The effect of the alert differed by drug, risk factor, specialty, and facility.
Conclusion: The delivery of evidence-based, patient-specific information had an influence on the final prescription in nearly 1 in 5 encounters. Our intervention was sustained with minimal prescriber fatigue over many years in a large and diverse health system.
Keywords: Clinical decision support; Clinical practice guideline; Decision-making; Implementation; Opioids.
© 2023. BioMed Central Ltd., part of Springer Nature.
Conflict of interest statement
Dr. Hsu reports consultancy and speaker fees for Stryker, consultancy and speaker fees from Smith & Nephew speakers’ bureau, speaker fees from Integra Lifesciences, and speaker fees from Depuy/Synthes. Dr. Bosse reports stock ownership in an orthopaedic implant company and grant funding from the Department of Defense. Dr. Griggs reports board membership for the American College of Emergency Physicians and payment from Boston University for preparation of pain management and opioid prescribing educational materials. Dr. Runyon reports research grant funding from Abbot Laboratories and Bristol-Myers Squibb and royalties/licenses from UpToDate. All remaining authors do not have any competing interests to declare.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

