Truncated Dyrk1A aggravates neuronal apoptosis by inhibiting ASF-mediated Bcl-x exon 2b inclusion
- PMID: 37864462
- PMCID: PMC11017436
- DOI: 10.1111/cns.14493
Truncated Dyrk1A aggravates neuronal apoptosis by inhibiting ASF-mediated Bcl-x exon 2b inclusion
Abstract
Aim: Aggravated neuronal loss, caused mainly by neuronal apoptosis, is observed in the brain of patients with Alzheimer's disease (AD) and animal models of AD. A truncated form of Dual-specific and tyrosine phosphorylation-regulated protein kinase 1A (Dyrk1A) plays a vital role in AD pathogenesis. Downregulation of anti-apoptotic Bcl-xL is tightly correlated with neuronal loss in AD. However, the molecular regulation of neuronal apoptosis and Bcl-x expression by Dyrk1A in AD remains largely elusive. Here, we aimed to explore the role and molecular mechanism of Dyrk1A in apoptosis.
Methods: Cell Counting Kit-8 (CCK8), flow cytometry, and TdT-mediated dUTP Nick-End Labeling (TUNEL) were used to check apoptosis. The cells, transfected with Dyrk1A or/and ASF with Bcl-x minigene, were used to assay Bcl-x expression by RT-PCR and Western blots. Co-immunoprecipitation, autoradiography, and immunofluorescence were conducted to check the interaction of ASF and Dyrk1A. Gene set enrichment analysis (GSEA) of apoptosis-related genes was performed in mice overexpressing Dyrk1A (TgDyrk1A) and AD model 5xFAD mice.
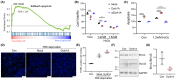
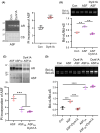
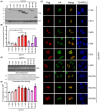
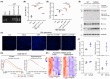
Results: Dyrk1A promoted Bcl-xS expression and apoptosis. Splicing factor ASF promoted Bcl-x exon 2b inclusion, leading to increased Bcl-xL expression. Dyrk1A suppressed ASF-mediated Bcl-x exon 2b inclusion via phosphorylation. The C-terminus deletion of Dyrk1A facilitated its binding and kinase activity to ASF. Moreover, Dyrk1a1-483 further suppressed the ASF-mediated Bcl-x exon 2b inclusion and aggravated apoptosis. The truncated Dyrk1A, increased Bcl-xS, and enrichment of apoptosis-related genes was observed in the brain of 5xFAD mice.
Conclusions: We speculate that increased Dyrk1A and truncated Dyrk1A may aggravate neuronal apoptosis by decreasing the ratio of Bcl-xL/Bcl-xS via phosphorylating ASF in AD.
Keywords: ASF; Bcl‐x; Dyrk1A; alternative splicing; apoptosis.
© 2023 The Authors. CNS Neuroscience & Therapeutics published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


Similar articles
-
Dual-specificity tyrosine phosphorylation-regulated kinase 1A promotes the inclusion of amyloid precursor protein exon 7.Biochem Pharmacol. 2024 Jun;224:116233. doi: 10.1016/j.bcp.2024.116233. Epub 2024 Apr 23. Biochem Pharmacol. 2024. PMID: 38663682
-
Link between DYRK1A overexpression and several-fold enhancement of neurofibrillary degeneration with 3-repeat tau protein in Down syndrome.J Neuropathol Exp Neurol. 2011 Jan;70(1):36-50. doi: 10.1097/NEN.0b013e318202bfa1. J Neuropathol Exp Neurol. 2011. PMID: 21157379 Free PMC article.
-
Truncation and Activation of Dual Specificity Tyrosine Phosphorylation-regulated Kinase 1A by Calpain I: A MOLECULAR MECHANISM LINKED TO TAU PATHOLOGY IN ALZHEIMER DISEASE.J Biol Chem. 2015 Jun 12;290(24):15219-37. doi: 10.1074/jbc.M115.645507. Epub 2015 Apr 27. J Biol Chem. 2015. PMID: 25918155 Free PMC article.
-
A review on synthetic inhibitors of dual-specific tyrosine phosphorylation-regulated kinase 1A (DYRK1A) for the treatment of Alzheimer's disease (AD).Bioorg Med Chem. 2024 Nov 1;113:117925. doi: 10.1016/j.bmc.2024.117925. Epub 2024 Sep 14. Bioorg Med Chem. 2024. PMID: 39357433 Review.
-
Leucettinib-21, a DYRK1A Kinase Inhibitor as Clinical Drug Candidate for Alzheimer's Disease and Down Syndrome.J Alzheimers Dis. 2024;101(s1):S95-S113. doi: 10.3233/JAD-240078. J Alzheimers Dis. 2024. PMID: 39422950 Review.
Cited by
-
A novel variant in GAS2 is associated with autosomal dominant nonsyndromic hearing impairment in a Chinese family.Hum Genomics. 2024 Jul 2;18(1):73. doi: 10.1186/s40246-024-00628-2. Hum Genomics. 2024. PMID: 38956677 Free PMC article.
References
-
- Bennett DA, Schneider JA, Wilson RS, Bienias JL, Arnold SE. Neurofibrillary tangles mediate the association of amyloid load with clinical Alzheimer disease and level of cognitive function. Arch Neurol. 2004;61(3):378‐384. - PubMed
-
- Braak H, Braak E, Grundke‐Iqbal I, Iqbal K. Occurrence of neuropil threads in the senile human brain and in Alzheimer's disease: a third location of paired helical filaments outside of neurofibrillary tangles and neuritic plaques. Neurosci Lett. 1986;65(3):351‐355. - PubMed
-
- Shimohama S. Apoptosis in Alzheimer's disease‐‐an update. Apoptosis. 2000;5(1):9‐16. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

