Responsive deep brain stimulation guided by ventral striatal electrophysiology of obsession durably ameliorates compulsion
- PMID: 37865084
- PMCID: PMC10841397
- DOI: 10.1016/j.neuron.2023.09.034
Responsive deep brain stimulation guided by ventral striatal electrophysiology of obsession durably ameliorates compulsion
Abstract
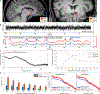
Treatment-resistant obsessive-compulsive disorder (OCD) occurs in approximately one-third of OCD patients. Obsessions may fluctuate over time but often occur or worsen in the presence of internal (emotional state and thoughts) and external (visual and tactile) triggering stimuli. Obsessive thoughts and related compulsive urges fluctuate (are episodic) and so may respond well to a time-locked brain stimulation strategy sensitive and responsive to these symptom fluctuations. Early evidence suggests that neural activity can be captured from ventral striatal regions implicated in OCD to guide such a closed-loop approach. Here, we report on a first-in-human application of responsive deep brain stimulation (rDBS) of the ventral striatum for a treatment-refractory OCD individual who also had comorbid epilepsy. Self-reported obsessive symptoms and provoked OCD-related distress correlated with ventral striatal electrophysiology. rDBS detected the time-domain area-based feature from invasive electroencephalography low-frequency oscillatory power fluctuations that triggered bursts of stimulation to ameliorate OCD symptoms in a closed-loop fashion. rDBS provided rapid, robust, and durable improvement in obsessions and compulsions. These results provide proof of concept for a personalized, physiologically guided DBS strategy for OCD.
Keywords: NAc-VeP; OCD; RNS; Y-BOCS; closed-loop fashion; detection; rDBS; time-domain feature; ventral striatum.
Copyright © 2023 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests No funding from NeuroPace was received for this study nor were data analyses reported here conducted by NeuroPace employees. C.H.H., R.S.S., and C.E.R. have patents related to sensing and brain stimulation for the treatment of neuropsychiatric disorders. C.H.H. is a consultant for Boston Scientific and Insightec and receives honoraria for educational lectures.
Figures


References
-
- Carmi L, Tendler A, Bystritsky A, Hollander E, Blumberger DM, Daskalakis J, Ward H, Lapidus K, Goodman W, Casuto L, et al. (2019). Efficacy and Safety of Deep Transcranial Magnetic Stimulation for Obsessive-Compulsive Disorder: A Prospective Multicenter Randomized Double-Blind Placebo-Controlled Trial. Am J Psychiatry 176, 931–938. 10.1176/appi.ajp.2019.18101180. - DOI - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

