Hematopoietic stem cells through the ages: A lifetime of adaptation to organismal demands
- PMID: 37865087
- PMCID: PMC10842631
- DOI: 10.1016/j.stem.2023.09.013
Hematopoietic stem cells through the ages: A lifetime of adaptation to organismal demands
Abstract
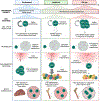
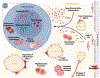
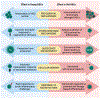
Hematopoietic stem cells (HSCs), which govern the production of all blood lineages, transition through a series of functional states characterized by expansion during fetal development, functional quiescence in adulthood, and decline upon aging. We describe central features of HSC regulation during ontogeny to contextualize how adaptive responses over the life of the organism ultimately form the basis for HSC functional degradation with age. We particularly focus on the role of cell cycle regulation, inflammatory response pathways, epigenetic changes, and metabolic regulation. We then explore how the knowledge of age-related changes in HSC regulation can inform strategies for the rejuvenation of old HSCs.
Keywords: aging; development; epigenetic; hematopoietic stem cells; inflammation; metabolism; niche regulation; quiescence; rejuvenation.
Copyright © 2023 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests E.P. is a member of the Cell Stem Cell advisory board.
Figures
References
-
- Boyd KL, and Bolon B (2022). Embryonic and Fetal Hematopoiesis. In Schalm’s Veterinary Hematology (John Wiley & Sons, Ltd; ), pp. 1–8. 10.1002/9781119500537.ch1. - DOI
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

