A highly specific antibody against the core fucose of the N-glycan in IgG identifies the pulmonary diseases and its regulation by CCL2
- PMID: 37865317
- PMCID: PMC10663832
- DOI: 10.1016/j.jbc.2023.105365
A highly specific antibody against the core fucose of the N-glycan in IgG identifies the pulmonary diseases and its regulation by CCL2
Abstract
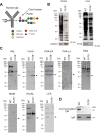
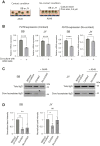
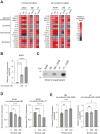
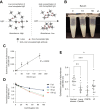
Glycan structure is often modulated in disease or predisease states, suggesting that such changes might serve as biomarkers. Here, we generated a monoclonal antibody (mAb) against the core fucose of the N-glycan in human IgG. Notably, this mAb can be used in Western blotting and ELISA. ELISA using this mAb revealed a low level of the core fucose of the N-glycan in IgG, suggesting that the level of acore fucosylated (noncore fucosylated) IgG was increased in the sera of the patients with lung cancer, chronic obstructive pulmonary disease, and interstitial pneumonia compared to healthy subjects. In a coculture analysis using human lung adenocarcinoma A549 cells and antibody-secreting B cells, the downregulation of the FUT8 (α1,6 fucosyltransferase) gene and a low level of core fucose of the N-glycan in IgG in antibody-secreting B cells were observed after coculture. A dramatic alteration in gene expression profiles for cytokines, chemokines, and their receptors were also observed after coculturing, and we found that the identified C-C motif chemokine 2 was partially involved in the downregulation of the FUT8 gene and the low level of core fucose of the N-glycan in IgG in antibody-secreting B cells. We also developed a latex turbidimetric immunoassay using this mAb. These results suggest that communication with C-C motif chemokine 2 between lung cells and antibody-secreting B cells downregulate the level of core fucose of the N-glycan in IgG, i.e., the increased level of acore fucosylated (noncore fucosylated) IgG, which would be a novel biomarker for the diagnosis of patients with pulmonary diseases.
Keywords: N-linked glycosylation; biomarker; chemokine; immunoglobulin G (IgG); lung.
Copyright © 2023 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest The authors declare no conflicts of interest with the contents of this article.
Figures

References
-
- Freeze H.H., Kinoshita T., Varki A. In: Essentials of Glycobiology. 3rd Ed. Varki A., Cummings R.D., Esko J.D., Stanley P., Hart G.W., Aebi M., et al., editors. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY: 2015. Glycans in acquired human diseases.
-
- Sackstein R., Hoffmeister K.M., Stowell S.R., Kinoshita T., Varki A., Freeze H.H. In: Essentials of Glycobiology. 4th Ed. Varki A., Cummings R.D., Esko J.D., Stanley P., Hart G.W., Aebi M., et al., editors. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY: 2022. Glycans in acquired human diseases.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

