Safety and efficacy of pegunigalsidase alfa in patients with Fabry disease who were previously treated with agalsidase alfa: results from BRIDGE, a phase 3 open-label study
- PMID: 37865771
- PMCID: PMC10589982
- DOI: 10.1186/s13023-023-02937-6
Safety and efficacy of pegunigalsidase alfa in patients with Fabry disease who were previously treated with agalsidase alfa: results from BRIDGE, a phase 3 open-label study
Abstract
Background: Pegunigalsidase alfa is a novel, PEGylated α-galactosidase-A enzyme-replacement therapy approved in the EU and US to treat patients with Fabry disease (FD).
Objective/methods: BRIDGE is a phase 3 open-label, switch-over study designed to assess safety and efficacy of 12 months of pegunigalsidase alfa (1 mg/kg every 2 weeks) treatment in adults with FD who had been previously treated with agalsidase alfa (0.2 mg/kg every 2 weeks) for ≥ 2 years.
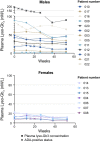
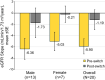
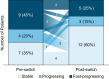
Results: Twenty-seven patients were screened; 22 met eligibility criteria; and 20 (13 men, 7 women) completed the study. Pegunigalsidase alfa was well-tolerated, with 97% of treatment-emergent adverse events (TEAEs) being of mild or moderate severity. The incidence of treatment-related TEAEs was low, with 2 (9%) discontinuations due to TEAEs. Five patients (23%) reported infusion-related reactions. Overall mean (SD; n = 22) baseline estimated glomerular filtration rate (eGFR) was 82.5 (23.4) mL/min/1.73 m2 and plasma lyso-Gb3 level was 38.3 (41.2) nmol/L (men: 49.7 [45.8] nmol/L; women: 13.8 [6.1] nmol/L). Before switching to pegunigalsidase alfa, mean (standard error [SE]) annualized eGFR slope was - 5.90 (1.34) mL/min/1.73 m2/year; 12 months post-switch, the mean eGFR slope was - 1.19 (1.77) mL/min/1.73 m2/year; and mean plasma lyso-Gb3 reduced by 31%. Seven (35%) out of 20 patients were positive for pegunigalsidase alfa antidrug antibodies (ADAs) at ≥ 1 study timepoint, two of whom had pre-existing ADAs at baseline. Mean (SE) changes in eGFR slope for ADA-positive and ADA-negative patients were + 5.47 (3.03) and + 4.29 (3.15) mL/min/1.73 m2/year, respectively, suggesting no negative impact of anti-pegunigalsidase alfa ADAs on eGFR slope.
Conclusion: Pegunigalsidase alfa may offer a safe and effective treatment option for patients with FD, including those previously treated with agalsidase alfa. TRN: NCT03018730. Date of registration: January 2017.
© 2023. Institut National de la Santé et de la Recherche Médicale (INSERM).
Conflict of interest statement
AL: Received speakers’ fees and consultancy honoraria from Chiesi, Sanofi-Genzyme, Takeda, Avrobio, and Alnylam. GD: Received speakers’ fees and consultancy honoraria from Sanofi, Takeda, Amicus, Chiesi, Greenovation Biotech GmbH. KN: Received grants and personal fees from Sanofi-Genzyme, Takeda; personal fees from Amicus, Idorsia, and Protalix. MW: Received research grants, honoraria, and/or consultant fees from Takeda, Sanofi-Genzyme, Amicus, Protalix, and Idorsia. Dr. West owns IP related to cardiac biomarkers and gene therapy in Fabry disease. CT: Received consultancy honoraria from Sanofi Genzyme, Amicus Therapeutics, Chiesi, and Acelink; participated in studies supported by Sanofi Genzyme, Shire, Protalix, Chiesi, Freeline, and Idorsia. AJ: Received grants from Amicus; honoraria and travel and accommodation from Takeda, Amicus, and Sanofi-Genzyme. PG: The author declares no competing interests. BV: Received speakers’ fees and consultancy honoraria from Sanofi-Genzyme, Takeda, Amicus, Chiesi, and Greenovation Biotech. TG: The author declares no competing interests. EB: Employed by Protalix. SA: Employed by Protalix. RC: Former full-time employee of Protalix Biotherapeutics and current consultant to Protalix Biotherapeutics and Chiesi USA, Inc. RR: Full-time employee of Chiesi Farmaceutici S.p.A. DH: Received honoraria for speaking and advisory boards from Amicus, Takeda, Sanofi-Genzyme, Freeline, and Protalix. Honoraria are administered through UCL consultants and are used in part to support laboratory research.
Figures
References
-
- Schiffmann R, Hughes DA, Linthorst GE, Ortiz A, Svarstad E, Warnock DG, et al. Screening, diagnosis, and management of patients with Fabry disease: conclusions from a "Kidney Disease: Improving Global Outcomes" (KDIGO) Controversies Conference. Kidney Int. 2017;91:284–293. - PubMed
-
- Wanner C, Arad M, Baron R, Burlina A, Elliott PM, Feldt-Rasmussen U, et al. European expert consensus statement on therapeutic goals in Fabry disease. Mol Genet Metab. 2018;124(3):189–203. - PubMed
-
- Germain DP, Weidemann F, Abiose A, Patel MR, Cizmarik M, Cole JA, et al. Analysis of left ventricular mass in untreated men and in men treated with agalsidase-β: data from the Fabry Registry. Genet Med. 2013;15(12):958–965. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

