Safety and tolerability of repeated doses of dihydroartemisinin-piperaquine for intermittent preventive treatment of malaria in pregnancy: a systematic review and an aggregated data meta-analysis of randomized controlled trials
- PMID: 37865784
- PMCID: PMC10590517
- DOI: 10.1186/s12936-023-04757-2
Safety and tolerability of repeated doses of dihydroartemisinin-piperaquine for intermittent preventive treatment of malaria in pregnancy: a systematic review and an aggregated data meta-analysis of randomized controlled trials
Abstract
Background: Malaria infection during pregnancy is an important cause of maternal and infant mortality and morbidity with the greatest effect being concentrated in sub-Saharan Africa. In areas of moderate to high malaria transmission, the World Health Organization (WHO) recommends the administration of intermittent preventive treatment of malaria in pregnancy (IPTp) using sulfadoxine-pyrimethamine (SP) to be given to all pregnant women at each scheduled antenatal care visit at monthly intervals. However, there is concern that increased resistance has compromised its effectiveness. This has led to a need for evaluation of alternatives to SP for IPTp with dihydroartemisinin-piperaquine (DP) emerging as a very promising candidate. Thus, this systematic review and aggregated data meta-analysis was conducted to establish the safety and tolerability of repeated doses with DP in IPTp.
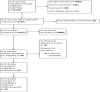
Methods: A systematic review and aggregated data meta-analysis of randomized controlled trials (RCTs) was performed by searching electronic databases of PubMed, Science Direct, ClinicalTrials.gov and Google Scholar. RCTs comparing IPTp DP versus recommended standard treatment for IPTp with these outcome measures were analyzed; change in QTc interval, serious adverse events (SAE), grade 3 or 4 adverse events possibly related to study drug and vomiting within 30 min after study drug administration. The search was performed up to 24th June 2023. Data was extracted from eligible studies and an aggregated data meta-analysis was carried out with data pooled as risk ratio (RR) with a 95% confidence interval (CI), using RevMan software (5.4). This study is registered with PROSPERO, CRD42022310041.
Results: Six RCTs involving 7969 participants were included in this systematic review and aggregated data meta-analysis. The pooled analysis showed that DP was associated with a change from baseline of the QTc interval although this change was not associated with cardiotoxicity. There was no statistically significant difference in the risk of occurrence of SAEs among participants in both treatment groups (RR = 0.80, 95% CI [0.52-1.24], P = 0.32). However, significant difference was observed in grade 3 or 4 AEs possibly related to study drug where analysis showed that subjects on IPT DP were statistically significantly more likely to experience an AE possibly related to study drug than subjects on IPT SP (RR = 6.65, 95% CI [1.18-37.54], P = 0.03) and in vomiting within 30 min after study drug administration where analysis showed that the risk of vomiting is statistically significantly higher in subjects receiving IPT DP than in subjects receiving IPT SP (RR = 1.77, 95% CI [1.02-3.07], P = 0.04).
Conclusion: DP was associated with a higher risk of grade 3 or 4 AEs possibly related to study drug and a higher risk of vomiting within 30 min after study drug administration. However, these were experienced in a very small percentage of women and did not affect adherence to study drugs. DP was also better tolerated in these studies as compared to most alternatives that have been proposed to replace SP which have proved to be too poorly tolerated in IPTp use.
Keywords: Aggregated data meta-analysis; Cardiotoxicity; Dihydroartemisinin-piperaquine; Intermittent preventive treatment; Malaria; Pregnancy; Randomised controlled trial; Safety; Tolerability.
© 2023. BioMed Central Ltd., part of Springer Nature.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


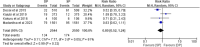

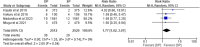
References
-
- Fasanya A, Mohammed N, Saleh BH, Tijani MK, Teleka A, Quintana MDP, et al. Anti-phosphatidylserine antibody levels are low in multigravid pregnant women in a malaria-endemic area in Nigeria, and do not correlate with anti-VAR2CSA antibodies. Front Cell Infect Microbiol. 2023;13:1130186. doi: 10.3389/fcimb.2023.1130186. - DOI - PMC - PubMed
-
- van Eijk AM, Hill J, Noor AM, Snow RW, ter Kuile FO. Prevalence of malaria infection in pregnant women compared with children for tracking malaria transmission in sub-Saharan Africa: a systematic review and meta-analysis. Lancet Glob Health. 2015;3:e617–e628. doi: 10.1016/S2214-109X(15)00049-2. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

