Activity-dependent glassy cell mechanics II: Nonthermal fluctuations under metabolic activity
- PMID: 37865819
- PMCID: PMC10698330
- DOI: 10.1016/j.bpj.2023.10.018
Activity-dependent glassy cell mechanics II: Nonthermal fluctuations under metabolic activity
Abstract
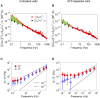
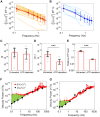
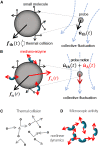
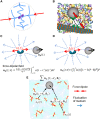
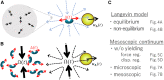
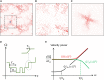
The glassy cytoplasm, crowded with bio-macromolecules, is fluidized in living cells by mechanical energy derived from metabolism. Characterizing the living cytoplasm as a nonequilibrium system is crucial in elucidating the intricate mechanism that relates cell mechanics to metabolic activities. In this study, we conducted active and passive microrheology in eukaryotic cells, and quantified nonthermal fluctuations by examining the violation of the fluctuation-dissipation theorem. The power spectral density of active force generation was estimated following the Langevin theory extended to nonequilibrium systems. However, experiments performed while regulating cellular metabolic activity showed that the nonthermal displacement fluctuation, rather than the active nonthermal force, is linked to metabolism. We discuss that mechano-enzymes in living cells do not act as microscopic objects. Instead, they generate meso-scale collective fluctuations with displacements controlled by enzymatic activity. The activity induces structural relaxations in glassy cytoplasm. Even though the autocorrelation of nonthermal fluctuations is lost at long timescales due to the structural relaxations, the nonthermal displacement fluctuation remains regulated by metabolic reactions. Our results therefore demonstrate that nonthermal fluctuations serve as a valuable indicator of a cell's metabolic activities, regardless of the presence or absence of structural relaxations.
Copyright © 2023 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures


References
-
- Haga H., Sasaki S., et al. Sambongi T. Elasticity mapping of living fibroblasts by AFM and immunofluorescence observation of the cytoskeleton. Ultramicroscopy. 2000;82:253–258. - PubMed
-
- Fabry B., Maksym G.N., et al. Fredberg J.J. Scaling the microrheology of living cells. Phys. Rev. Lett. 2001;87 - PubMed
-
- Fabry B., Maksym G.N., et al. Fredberg J.J. Time scale and other invariants of integrative mechanical behavior in living cells. Phys. Rev. E - Stat. Nonlinear Soft Matter Phys. 2003;68(4 Pt 1) - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

