Prevalent bee venom genes evolved before the aculeate stinger and eusociality
- PMID: 37867198
- PMCID: PMC10591384
- DOI: 10.1186/s12915-023-01656-5
Prevalent bee venom genes evolved before the aculeate stinger and eusociality
Abstract
Background: Venoms, which have evolved numerous times in animals, are ideal models of convergent trait evolution. However, detailed genomic studies of toxin-encoding genes exist for only a few animal groups. The hyper-diverse hymenopteran insects are the most speciose venomous clade, but investigation of the origin of their venom genes has been largely neglected.
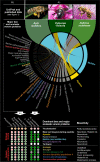
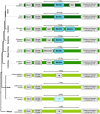
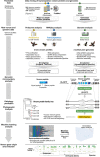
Results: Utilizing a combination of genomic and proteo-transcriptomic data, we investigated the origin of 11 toxin genes in 29 published and 3 new hymenopteran genomes and compiled an up-to-date list of prevalent bee venom proteins. Observed patterns indicate that bee venom genes predominantly originate through single gene co-option with gene duplication contributing to subsequent diversification.
Conclusions: Most Hymenoptera venom genes are shared by all members of the clade and only melittin and the new venom protein family anthophilin1 appear unique to the bee lineage. Most venom proteins thus predate the mega-radiation of hymenopterans and the evolution of the aculeate stinger.
Keywords: Aculeatoxins; Apamin; Bee toxins; Genomics; Hymenoptera venom; Machine learning; Melittin; Proteo-transcriptomics; Solitary bee venom; Venom gene evolution.
© 2023. BioMed Central Ltd., part of Springer Nature.
Conflict of interest statement
The authors declare they have no competing interests.
Figures






References
-
- Casewell NR, Wüster W, Vonk FJ, Harrison RA, Fry BG. Complex cocktails: the evolutionary novelty of venoms. Trends Ecol Evol. 2013;28(4):219–229. - PubMed
-
- Wang T, Zhao M, Rotgans BA, Ni G, Dean JFD, Nahrung HF, et al. Proteomic analysis of the venom and venom sac of the woodwasp, Sirex noctilio - towards understanding its biological impact. J Proteomics. 2016;146:195–206. - PubMed
-
- Piek T. Venoms of the Hymenoptera. London: Academic Press Inc. (London) Ltd.; 1986.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

