Actomyosin Activity and Piezo1 Activity Synergistically Drive Urinary System Fibroblast Activation
- PMID: 37867255
- PMCID: PMC10667826
- DOI: 10.1002/advs.202303369
Actomyosin Activity and Piezo1 Activity Synergistically Drive Urinary System Fibroblast Activation
Abstract
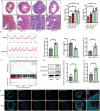
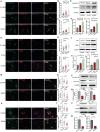
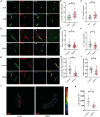
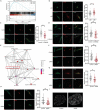
Mechanical cues play a crucial role in activating myofibroblasts from quiescent fibroblasts during fibrosis, and the stiffness of the extracellular matrix is of significant importance in this process. While intracellular force mediated by myosin II and calcium influx regulated by Piezo1 are the primary mechanisms by which cells sense and respond to mechanical forces, their intercellular mechanical interaction remains to be elucidated. Here, hydrogels with tunable substrate are used to systematically investigate the crosstalk of myosin II and Piezo1 in fibroblast to myofibroblast transition (FMT). The findings reveal that the two distinct signaling pathways are integrated to convert mechanical stiffness signals into biochemical signals during bladder-specific FMT. Moreover, it is demonstrated that the crosstalk between myosin II and Piezo1 sensing mechanisms synergistically establishes a sustained feed-forward loop that contributes to chromatin remodeling, induces the expression of downstream target genes, and ultimately exacerbates FMT, in which the intracellular force activates Piezo1 by PI3K/PIP3 pathway-mediated membrane tension and the Piezo1-regulated calcium influx enhances intracellular force by the classical FAK/RhoA/ROCK pathway. Finally, the multifunctional Piezo1 in the complex feedback circuit of FMT drives to further identify that targeting Piezo1 as a therapeutic option for ameliorating bladder fibrosis and dysfunction.
Keywords: Piezo1; actomyosin; fibroblasts; fibrosis; urinary Systems.
© 2023 The Authors. Advanced Science published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Friedman S. L., Sheppard D., Duffield J. S., Violette S., Sci. Transl. Med. 2013, 5, 167sr1. - PubMed
-
- Rockey D. C., Bell P. D., Hill J. A., N. Engl. J. Med. 2015, 372, 1138. - PubMed
-
- Wang W., Ai J., Liao B., Xiao K., Lin L., Chen H., Zhou L., Int. Immunopharmacol. 2021, 99, 107947. - PubMed
-
- Bushman W. A., Jerde T. J., Am. J. Physiol. Renal. Physiol. 2016, 311, F817. - PubMed
-
- Akino H., Gobara M., Okada K., Int. J. Urol. 1996, 3, 441. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
