Design and Synthesis of Ketenimine Sulfonamide Conjugates through Multicomponent Reactions; A Combined Cytotoxic Analysis and Computational Exploration
- PMID: 37867708
- PMCID: PMC10586297
- DOI: 10.1021/acsomega.3c05816
Design and Synthesis of Ketenimine Sulfonamide Conjugates through Multicomponent Reactions; A Combined Cytotoxic Analysis and Computational Exploration
Erratum in
-
Correction to "Design and Synthesis of Ketenimine Sulfonamide Conjugates through Multicomponent Reactions; A Combined Cytotoxic Analysis and Computational Exploration".ACS Omega. 2023 Nov 29;8(49):47315. doi: 10.1021/acsomega.3c08691. eCollection 2023 Dec 12. ACS Omega. 2023. PMID: 38107917 Free PMC article.
Abstract
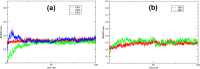
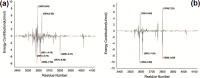
Multicomponent reactions involving zwitterion generated from dimethyl acetylenedicarboxylate, aryl sulfonamide, and isocyanide to generate sulfonamide-conjugated ketenimines is reported. The synthetic strategy adopted is highly atom economical and stereoselective. Ketenimine sulfonamide analogues are key intermediates for further synthetic conversions to generate a combinatorial library of compounds. Furthermore, sulfonamide compounds are known to possess a broad spectrum of biological applications. All the novel molecules synthesized exhibit the potential to target the nonhomologous DNA end-joining (NHEJ) pathway with cytotoxic ability. Computational studies compliment the in vitro biological assays of the 8 small-molecule inhibitors. DNA double-strand breaks (DSBs) are considered as the most lethal among different DNA damages. NHEJ repairs about 70% of the DSBs generated in cells within mammals. The DNA-dependent protein kinase catalytic subunit is one of the PI3 kinases associated with NHEJ. Compounds DK01-DK08 were investigated for their ability to induce cancer cell death by treating with two leukemic cell lines where NHEJ is high. Results showed that bromoaryl (DK04)- and nitroaryl (DK05)-conjugated molecules showed excellent biological activity, having IC50 values of ∼2 μM in Nalm6 cell lines.
© 2023 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures











References
-
- Jemal A.; Thun M. J.; Ries L. A. G.; Howe H. L.; Weir H. K.; Center M. M.; Ward E.; Wu X.-C.; Eheman C.; Anderson R.; Ajani U. A.; Kohler B.; Edwards B. K. Annual Report to the Nation on the Status of Cancer, 1975–2005, Featuring Trends in Lung Cancer, Tobacco Use, and Tobacco Control. JNCI, J. Natl. Cancer Inst. 2008, 100 (23), 1672–1694. 10.1093/jnci/djn389. - DOI - PMC - PubMed
-
- Ray U.; John F.; Pooppadi S.; George J.; Sharma S.; Raghavan S. C. Novel Synthetic Aromatic Thiourea Derivatives and Investigations on Their Cytotoxic Potential Efficacy. J. Heterocycl. Chem. 2021, 58 (1), 40–47. 10.1002/jhet.4145. - DOI
LinkOut - more resources
Full Text Sources

