Newly Synthesized Anticancer Purine Derivatives Inhibiting p-EIF4E Using Surface-Modified Lipid Nanovesicles
- PMID: 37867723
- PMCID: PMC10586017
- DOI: 10.1021/acsomega.3c02991
Newly Synthesized Anticancer Purine Derivatives Inhibiting p-EIF4E Using Surface-Modified Lipid Nanovesicles
Abstract
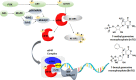
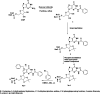
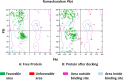
Translation of mRNA is one of the processes adopted by cancer cells to maintain survival via phosphorylated (p)-eIF4E overexpression. Once p-eIF4E binds to the cap structure of mRNA, it advocates a nonstop translation process. In this regard, 15 new-based GMP analogs were synthesized to target eIF4E and restrain its binding to cap mRNA. The compounds were tested against three types of cancer cell lines: Caco-2, HepG-2, MCF-7, and normal kidney cells (Vero cells). Most of the compounds showed high potency against breast cancer cells (MCF-7), characterized by the highest cancer type for overexpression of p-eIF4E. Compound 4b was found to be the most active against three cell lines, colon (Caco-2), hepatic (HepG-2), and breast (MCF-7), with positive IC50 values of 31.40, 27.15, and 21.71 μM, respectively. Then, chitosan-coated niosomes loaded with compound 4b (Cs/4b-NSs) were developed (as kinetically enhanced molecules) to improve the anticancer effects further. The prepared Cs/4b-NSs showed pronounced cytotoxicity compared to the free 4b against Caco2, Hepg2, and MCF-7 with IC50 values of 16.15, 26.66, and 6.90 μM, respectively. Then, the expression of both the phosphorylated and nonphosphorylated western blot techniques was conducted on MCF-7 cells treated with the most active compounds (based on the obtained IC50 values) to determine the total protein expression of both eIF4E and p-eIF4e. Interestingly, the selected most active compounds displayed 35.8-40.7% inhibition of p-eIF4E expression when evaluated on MCF-7 compared to Ribavirin (positive control). CS/4b-NSs showed the best inhibition (40.7%). The findings of the present joint in silico molecular docking, simulation dynamic studies, and experimental investigation suggest the potential use of niosomal nanovesicles as a promising nanocarrier for the targeted delivery of the newly synthesized compound 4b to eukaryotic initiation factor 4E. These outcomes support the possible use of Cs/4b-NSs in targeted cancer therapy.
© 2023 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures





References
LinkOut - more resources
Full Text Sources
Miscellaneous

