Low levels of endogenous anabolic androgenic steroids in females with severe asthma taking corticosteroids
- PMID: 37868143
- PMCID: PMC10588792
- DOI: 10.1183/23120541.00269-2023
Low levels of endogenous anabolic androgenic steroids in females with severe asthma taking corticosteroids
Abstract
Rationale: Patients with severe asthma are dependent upon treatment with high doses of inhaled corticosteroids (ICS) and often also oral corticosteroids (OCS). The extent of endogenous androgenic anabolic steroid (EAAS) suppression in asthma has not previously been described in detail. The objective of the present study was to measure urinary concentrations of EAAS in relation to exogenous corticosteroid exposure.
Methods: Urine collected at baseline in the U-BIOPRED (Unbiased Biomarkers for the Prediction of Respiratory Disease outcomes) study of severe adult asthmatics (SA, n=408) was analysed by quantitative mass spectrometry. Data were compared to that of mild-to-moderate asthmatics (MMA, n=70) and healthy subjects (HC, n=98) from the same study.
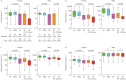
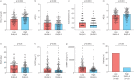
Measurements and main results: The concentrations of urinary endogenous steroid metabolites were substantially lower in SA than in MMA or HC. These differences were more pronounced in SA patients with detectable urinary OCS metabolites. Their dehydroepiandrosterone sulfate (DHEA-S) concentrations were <5% of those in HC, and cortisol concentrations were below the detection limit in 75% of females and 82% of males. The concentrations of EAAS in OCS-positive patients, as well as patients on high-dose ICS only, were more suppressed in females than males (p<0.05). Low levels of DHEA were associated with features of more severe disease and were more prevalent in females (p<0.05). The association between low EAAS and corticosteroid treatment was replicated in 289 of the SA patients at follow-up after 12-18 months.
Conclusion: The pronounced suppression of endogenous anabolic androgens in females might contribute to sex differences regarding the prevalence of severe asthma.
Copyright ©The authors 2023.
Conflict of interest statement
Conflict of interest: V. Yasinska reports participation in advisory boards for AZ and GSK, and lecture honoraria from Sanofi and GSK. Conflict of interest: C. Gómez has nothing to disclose. Conflict of interest: J. Kolmert has nothing to disclose. Conflict of interest: M. Ericsson has nothing to disclose. Conflict of interest: A. Pohanka has nothing to disclose. Conflict of interest: A. James reports personal grant from Swedish Heart-Lung Foundation. Conflict of interest: L.I. Andersson has nothing to disclose. Conflict of interest: M. Sparreman-Mikus has nothing to disclose. Conflict of interest: A.R. Sousa reports employment and stocks or stock options from GSK. Conflict of interest: J.H. Riley has nothing to disclose. Conflict of interest: S. Bates has nothing to disclose. Conflict of interest: P.S. Bakke reports lecture honoraria from AstraZeneca and Boehringer Ingelheim. Conflict of interest: N. Zounemat Kermani has nothing to disclose. Conflict of interest: M. Caruso has nothing to disclose. Conflict of interest: P. Chanez reports participation in advisory boards, honoraria for consultancy, lectures fees and support for attending and/or travel from ALK, Almirall, AZ, Chiesi, GSK, Menarini, Novartis and Sanofi. Conflict of interest: S.J. Fowler has nothing to disclose. Conflict of interest: T. Geiser has nothing to disclose. Conflict of interest: P.H. Howarth reports employment and stocks or stock options from GSK. Conflict of interest: I. Horváth reports participation on an advisory board for AZ and Chiesi, honoraria for lectures from Chiesi and Roche, and support for attending and/or travel from Roche. Conflict of interest: N. Krug has nothing to disclose. Conflict of interest: P. Montuschi has nothing to disclose. Conflict of interest: M. Sanak has nothing to disclose. Conflict of interest: A. Behndig has nothing to disclose. Conflict of interest: D.E. Shaw has nothing to disclose. Conflict of interest: R.G. Knowles has nothing to disclose. Conflict of interest: B. Dahlén reports grant from GSK and Novartis. Conflict of interest: A-H. Maitland-van der Zee reports grants from BI, Vertex Innovation Award, Dutch Lung Foundation, Stichting Astma Bestrijding, IMI/3TR, EU grant ONELAB and EUROSTARS grant with Respiq, consulting fees from AZ and BI, and lecture honoraria from GSK. Conflict of interest: P.J. Sterk reports a grant from the Innovative Medicines Initiative. Conflict of interest: R. Djukanovic reports consulting fees from Synairgen, lecture honoraria from Regeneron, GSK and Kymab, and an advisory board for Synairgen. Conflict of interest: I.M. Adcock reports grant from EU-IMI, grants from GSK, MRK, EPSRC and Sanofi, consulting fees from GSK, Sanofi, Chiesi and Kinaset, lecture honoraria from AZ, Sanofi and Eurodrug, and payment for an educational event from Sunovion. Conflict of interest: K.F. Chung reports lectures honoraria from Novartis, AZ and Merck, advisory boards for GSK, AZ, Novartis, Roche, Merck, Rickett-Beckinson, Nocion and Shionogi, the Scientific Advisory Board of the Clean Breathing Institute supported by Haleon, grants from GSK, MRC and EPSRC, and support for travel from AZ. Conflict of interest: C.E. Wheelock has nothing to disclose. Conflict of interest: S-E. Dahlén reports research grants, consulting fees or lecture honoraria from AZ, Cayman Chemicals, GSK, Regeneron, Sanofi and Teva. Conflict of interest: E. Wikström Jonsson reports a research grant and expert assignment by Region Stockholm, and an expert appointment by the Swedish Medical Product Agency.
Figures


References
LinkOut - more resources
Full Text Sources
