Effects of bepotastine, a nonsedating H1-antihistamine, for the treatment of persistent cough and allergic rhinitis: a randomised, double-blind, placebo-controlled trial
- PMID: 37868148
- PMCID: PMC10588790
- DOI: 10.1183/23120541.00448-2023
Effects of bepotastine, a nonsedating H1-antihistamine, for the treatment of persistent cough and allergic rhinitis: a randomised, double-blind, placebo-controlled trial
Abstract
Background: Empirical therapy with oral histamine-1 receptor antagonists (H1RAs) is often used for patients with suspected upper airway cough syndrome. No placebo-controlled trials with nonsedating H1RAs (nsH1RAs) have evaluated validated cough outcomes. The objective of the present study was to assess the effect of an nsH1RA, bepotastine, on cough outcomes in patients with allergic rhinitis and persistent cough.
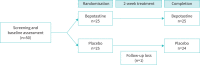
Methods: A randomised, double-blind, placebo-controlled trial was conducted. Adult patients with persistent cough (>3 weeks in duration) and symptomatic allergic rhinitis were recruited and randomly assigned to receive either bepotastine or placebo at a 1:1 ratio. The primary outcome was cough-specific quality of life assessed using the Leicester Cough Questionnaire (LCQ). Secondary outcomes included cough severity visual analogue scale (VAS), throat VAS, Cough Hypersensitivity Questionnaire, Sinonasal Outcome Test-22 score and drug adverse events.
Results: Between October 2021 and September 2022, 50 participants (43 females; mean age 46.28 years; median cough duration 3 months) were assigned to either the bepotastine 10 mg twice daily or placebo group in a 1:1 ratio. After 2 weeks of treatment, both bepotastine and placebo groups showed significant improvements in the LCQ scores, but there was no significant difference in the magnitude of change between the groups (3.45±2.10 versus 3.04±2.94, p=0.576). Secondary outcomes were also comparable.
Conclusions: Despite the relatively small sample size, our study clearly demonstrated that a 2-week treatment with bepotastine did not provide therapeutic benefits for cough outcomes. These findings suggest against the use of nsH1RAs with the intention of improving cough outcomes, even in patients with persistent cough and allergic rhinitis.
Copyright ©The authors 2023.
Conflict of interest statement
Conflict of interest: W-J. Song is deputy chief editor of this journal. The other authors declare that they have no competing interests.
Figures


Similar articles
-
Double-blind placebo-controlled study of bepotastine besilate in pediatric patients with perennial allergic rhinitis.Expert Opin Pharmacother. 2015;16(16):2395-408. doi: 10.1517/14656566.2015.1085511. Epub 2015 Sep 12. Expert Opin Pharmacother. 2015. PMID: 26364765 Clinical Trial.
-
Efficacy of non-sedating H1-receptor antihistamines in adults and adolescents with chronic cough: A systematic review.World Allergy Organ J. 2021 Jul 21;14(8):100568. doi: 10.1016/j.waojou.2021.100568. eCollection 2021 Aug. World Allergy Organ J. 2021. PMID: 34386152 Free PMC article.
-
Time to onset and duration of action of the antihistamine bepotastine besilate ophthalmic solutions 1.0% and 1.5% in allergic conjunctivitis: a phase III, single-center, prospective, randomized, double-masked, placebo-controlled, conjunctival allergen challenge assessment in adults and children.Clin Ther. 2009 Sep;31(9):1908-21. doi: 10.1016/j.clinthera.2009.09.001. Clin Ther. 2009. PMID: 19843481 Clinical Trial.
-
Oral bepotastine: in allergic disorders.Drugs. 2010 Aug 20;70(12):1579-91. doi: 10.2165/11205880-000000000-00000. Drugs. 2010. PMID: 20687621 Review.
-
Speech and language therapy for management of chronic cough.Cochrane Database Syst Rev. 2019 Jul 23;7(7):CD013067. doi: 10.1002/14651858.CD013067.pub2. Cochrane Database Syst Rev. 2019. PMID: 31335963 Free PMC article.
Cited by
-
How Will a Treatable Traits Approach Reshape Clinical Practice in Chronic Cough?Allergy Asthma Immunol Res. 2025 May;17(3):304-316. doi: 10.4168/aair.2025.17.3.304. Allergy Asthma Immunol Res. 2025. PMID: 40414808 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
