A five-antigen Esx-5a fusion delivered as a prime-boost regimen protects against M.tb challenge
- PMID: 37869008
- PMCID: PMC10585038
- DOI: 10.3389/fimmu.2023.1263457
A five-antigen Esx-5a fusion delivered as a prime-boost regimen protects against M.tb challenge
Abstract
The development of tuberculosis (TB) vaccines has been hindered by the complex nature of Mycobacterium tuberculosis (M.tb) and the absence of clearly defined immune markers of protection. While Bacillus Calmette-Guerin (BCG) is currently the only licensed TB vaccine, its effectiveness diminishes in adulthood. In our previous research, we identified that boosting BCG with an intranasally administered chimpanzee adenovirus expressing the PPE15 antigen of M.tb (ChAdOx1.PPE15) improved its protection. To enhance the vaccine's efficacy, we combined PPE15 with the other three members of the Esx-5a secretion system and Ag85A into a multi-antigen construct (5Ag). Leveraging the mucosal administration safety of ChAdOx1, we targeted the site of M.tb infection to induce localized mucosal responses, while employing modified vaccinia virus (MVA) to boost systemic immune responses. The combination of these antigens resulted in enhanced BCG protection in both the lungs and spleens of vaccinated mice. These findings provide support for advancing ChAdOx1.5Ag and MVA.5Ag to the next stages of vaccine development.
Keywords: BCG; Esx; mucosa; protection; subunit; tuberculosis; vaccines; viral-vector.
Copyright © 2023 Stylianou, Pinpathomrat, Sampson, Richard, Korompis and McShane.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

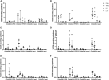
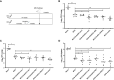



References
-
- World Health Organisation (WHO) . Global TB report 2022. (2021).
-
- Fine PCI, Milstien J, Clements CJ. Issues relating to the use of BCG in immunisation programmes: A discussion document. World Health Organisation; (1999). Available at: https://iris.who.int/bitstream/handle/10665/66120/WHO_V_B_99.23.pdf?sequ....
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

