Multifaceted roles and regulation of nucleotide-binding oligomerization domain containing proteins
- PMID: 37869013
- PMCID: PMC10585062
- DOI: 10.3389/fimmu.2023.1242659
Multifaceted roles and regulation of nucleotide-binding oligomerization domain containing proteins
Abstract
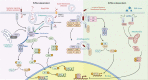
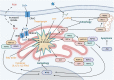
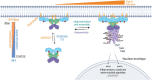
Nucleotide-binding oligomerization domain-containing proteins, NOD1 and NOD2, are cytosolic receptors that recognize dipeptides and tripeptides derived from the bacterial cell wall component peptidoglycan (PGN). During the past two decades, studies have revealed several roles for NODs beyond detecting PGN fragments, including activation of an innate immune anti-viral response, NOD-mediated autophagy, and ER stress induced inflammation. Recent studies have also clarified the dynamic regulation of NODs at cellular membranes to generate specific and balanced immune responses. This review will describe how NOD1 and NOD2 detect microbes and cellular stress and detail the molecular mechanisms that regulate activation and signaling while highlighting new evidence and the impact on inflammatory disease pathogenesis.
Keywords: Crohn’s; NF-κB; NOD1; NOD2; inflammation; peptidoglycan.
Copyright © 2023 Dixon, Wu and Fairn.
Conflict of interest statement
The authors declare that the manuscript was written in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

