Curcumin Transferosome-Loaded Thermosensitive Intranasal in situ Gel as Prospective Antiviral Therapy for SARS-Cov-2
- PMID: 37869062
- PMCID: PMC10590117
- DOI: 10.2147/IJN.S423251
Curcumin Transferosome-Loaded Thermosensitive Intranasal in situ Gel as Prospective Antiviral Therapy for SARS-Cov-2
Abstract
Purpose: Immunomodulatory and broad-spectrum antiviral activities have motivated the evaluation of curcumin for Coronavirus infection 2019 (COVID-19) management. Inadequate bioavailability is the main impediment to the therapeutic effects of oral Cur. This study aimed to develop an optimal curcumin transferosome-loaded thermosensitive in situ gel to improve its delivery to the lungs.
Methods: Transferosomes were developed by using 33 screening layouts. The phospholipid concentration as well as the concentration and type of surfactant were considered independent variables. The entrapment efficiency (EE%), size, surface charge, and polydispersity index (PDI) were regarded as dependent factors. A cold technique was employed to develop thermosensitive in-situ gels. Optimized transferosomes were loaded onto the selected gels. The produced gel was assessed based on shape attributes, ex vivo permeability enhancement, and the safety of the nasal mucosa. The in vitro cytotoxicity, antiviral cytopathic effect, and plaque assay (CV/CPE/Plaque activity), and in vivo performance were evaluated after intranasal administration in experimental rabbits.
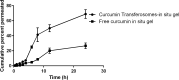
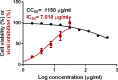
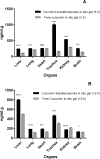
Results: The optimized preparation displayed a particle size of 664.3 ± 69.3 nm, EE% of 82.8 ± 0.02%, ZP of -11.23 ± 2.5 mV, and PDI of 0.6 ± 0.03. The in vitro curcumin release from the optimized transferosomal gel was markedly improved compared with that of the free drug-loaded gel. An ex vivo permeation study revealed a significant improvement (2.58-fold) in drug permeability across nasal tissues of sheep. Histopathological screening confirmed the safety of these preparations. This formulation showed high antiviral activity against SARS-CoV-2 at reduced concentrations. High relative bioavailability (226.45%) was attained after the formula intranasally administered to rabbits compared to the free drug in-situ gel. The curcumin transferosome gel displayed a relatively high lung accumulation after intranasal administration.
Conclusion: This study provides a promising formulation for the antiviral treatment of COVID-19 patients, which can be evaluated further in preclinical and clinical studies.
Keywords: SARS-CoV-2; coronavirus 2; curcumin; in situ gels; intranasal delivery; transferosomes.
© 2023 Eleraky et al.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could influence the work reported in this study.
Figures






References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

