The effect of cartilage dehydration and rehydration on quantitative ultrashort echo time biomarkers
- PMID: 37869338
- PMCID: PMC10585582
- DOI: 10.21037/qims-23-359
The effect of cartilage dehydration and rehydration on quantitative ultrashort echo time biomarkers
Abstract
Background: The effect of dehydration of ex vivo cartilage samples and rehydration with native synovial fluid or normal saline on quantitative ultrashort echo time (UTE) biomarkers are unknown. We aimed to investigate the effect of cartilage dehydration-rehydration on UTE biomarkers and to compare the rehydration capabilities of native synovial fluid and normal saline.
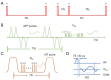
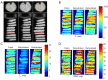
Methods: A total of 37 cartilage samples were harvested from patients (n=5) who underwent total knee replacement. Fresh cartilage samples were exposed to air to dehydrate for 2 hours after baseline magnetic resonance (MR) scanning, then randomly divided into two groups: one soaking in native synovial fluid (n=17) and the other in normal saline (n=20) to rehydrate for 4 hours. UTE-based biomarkers [T1, adiabatic T1r (AdiabT1r), macromolecular fraction (MMF), magnetization transfer ratio (MTR), and T2*] and sample weights were evaluated for fresh, dehydrated, and rehydrated cartilage samples. Differences and agreements between groups were assessed using the values of fresh cartilage samples as reference standard.
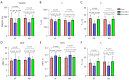
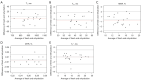
Results: Dehydrating in air for 2 hours resulted in significant weight loss (P=0.000). T1, AdiabT1r, and T2* decreased significantly while MMF and MTR increased significantly (all P<0.02). Non-significant differences were observed in cartilage weights after rehydrating in both synovial fluid and normal saline, with P values being 0.204 and 0.769, respectively. There were no significant differences in T1, AdiabT1r, MMF, and MTR after rehydrating in synovial fluid (P>0.0167, with Bonferroni correction) while T2* (P=0.001) still had significant differences compared with fresh samples. However, no significant differences were detected for any of the evaluated UTE biomarkers after rehydrating in normal saline (all P>0.05). No differences were detected in the agreement of UTE biomarker measurements between fresh samples and samples rehydrated with synovial fluid and normal saline.
Conclusions: Cartilage dehydration resulted in significant changes in UTE biomarkers. Rehydrating with synovial fluid or normal saline had non-significant effect on all the evaluated UTE biomarkers except T2* values, which still had significant differences compared with fresh samples after rehydrating with synovial fluid. No significant difference was observed in the rehydration capabilities of native synovial fluid and normal saline.
Keywords: Cartilage; dehydration; fresh; rehydration; ultrashort echo time magnetic resonance imaging (UTE-MRI).
2023 Quantitative Imaging in Medicine and Surgery. All rights reserved.
Conflict of interest statement
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-23-359/coif). JD serves as an unpaid editorial board member of Quantitative Imaging in Medicine and Surgery. The other authors have no conflicts of interest to declare.
Figures