Systematic characterization of antibody-drug conjugate targets in central nervous system tumors
- PMID: 37870091
- PMCID: PMC10912007
- DOI: 10.1093/neuonc/noad205
Systematic characterization of antibody-drug conjugate targets in central nervous system tumors
Abstract
Background: Antibody-drug conjugates (ADCs) enhance the specificity of cytotoxic drugs by directing them to cells expressing target antigens. Multiple ADCs are FDA-approved for solid and hematologic malignancies, including those expressing HER2, TROP2, and NECTIN4. Recently, an ADC targeting HER2 (Trastuzumab-Deruxtecan) increased survival and reduced growth of brain metastases in treatment-refractory metastatic breast cancer, even in tumors with low HER2 expression. Thus, low-level expression of ADC targets may be sufficient for treatment responsiveness. However, ADC target expression is poorly characterized in many central nervous system (CNS) tumors.
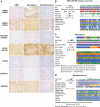
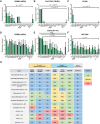
Methods: We analyzed publicly available RNA-sequencing and proteomic data from the children's brain tumor network (N = 188 tumors) and gene-expression-omnibus RNA-expression datasets (N = 356) to evaluate expression of 14 potential ADC targets that are FDA-approved or under investigation in solid cancers. We also used immunohistochemistry to measure the levels of HER2, HER3, NECTIN4, TROP2, CLDN6, CLDN18.2, and CD276/B7-H3 protein in glioblastoma, oligodendroglioma, meningioma, ependymoma, pilocytic astrocytoma, medulloblastoma, atypical teratoid/rhabdoid tumor (AT/RT), adamantinomatous craniopharyngioma (ACP), papillary craniopharyngioma (PCP), and primary CNS lymphoma (N = 575).
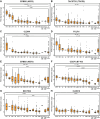
Results: Pan-CNS analysis showed subtype-specific expression of ADC target proteins. Most tumors expressed HER3, B7-H3, and NECTIN4. Ependymomas strongly expressed HER2, while meningiomas showed weak-moderate HER2 expression. ACP and PCP strongly expressed B7-H3, with TROP2 expression in whorled ACP epithelium. AT/RT strongly expressed CLDN6. Glioblastoma showed little subtype-specific marker expression, suggesting a need for further target development.
Conclusions: CNS tumors exhibit subtype-specific expression of ADC targets including several FDA-approved for other indications. Clinical trials of ADCs in CNS tumors may therefore be warranted.
Keywords: craniopharyngioma; ependymoma; glioma; immunoconjugates; meningioma.
© The Author(s) 2023. Published by Oxford University Press on behalf of the Society for Neuro-Oncology. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Conflict of interest statement
K.L.L. has received research support from Dana-Farber Cancer Institute from Rarecyte and Bristol Myers Squibb, consulting fees from BMS, Integragen, Blaze Biosciences, and Travera Inc., and is an equity holder and founder of Travera Inc. P.K.S. is a co-founder and member of the Board of Directors of Glencoe Software, a member of the Board of Directors for Applied Biomath, and a member of the Scientific Advisory Board for RareCyte, NanoString, and Montai Health; he holds equity in Glencoe, Applied Biomath, and RareCyte. P.K.S. is a consultant for Merck and the Sorger lab has received research funding from Novartis and Merck in the past five years. P.Y.W. has received honoraria for consulting from Astra Zeneca, Bayer, Black Diamond, Boehringer Ingelheim, Boston Pharmaceuticals, Celularity, Chimerix, Day One Bio, Genenta, Glaxo Smith Kline, Karyopharm, Merck, Mundipharma, Novartis, Novocure, Nuvation Bio, Prelude Therapeutics, Sapience, Servier, Sagimet, Vascular Biogenics, and VBI Vaccines.
Figures



References
-
- Louis DN, Ohgaki H, Wiestler OD, et al. WHO Classification of Tumours of the Central Nervous System. 6th ed. Lyon: International Agency for Research on Cancer; 2021.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

