Immunoglobulin treatment for hospitalised infants and young children with respiratory syncytial virus infection
- PMID: 37870128
- PMCID: PMC10591280
- DOI: 10.1002/14651858.CD009417.pub3
Immunoglobulin treatment for hospitalised infants and young children with respiratory syncytial virus infection
Abstract
Background: Millions of children are hospitalised due to respiratory syncytial virus (RSV) infection every year. Treatment is supportive, and current therapies (e.g. inhaled bronchodilators, epinephrine, nebulised hypertonic saline, and corticosteroids) are ineffective or have limited effect. Respiratory syncytial virus immunoglobulin may be used prophylactically to prevent hospital admission from RSV-related illness. It may be considered for the treatment of established severe RSV infection or for treatment in an immunocompromised host, although it is not licensed for this purpose. It is unclear whether immunoglobulins improve outcomes when used as a treatment for established RSV infection in infants and young children admitted to hospital. This is an update of a review first published in 2019.
Objectives: To assess the effects of immunoglobulins for the treatment of RSV-proven lower respiratory tract infections (LRTIs) in children aged up to three years, admitted to hospital.
Search methods: For this 2022 update, we searched the Cochrane Central Register of Controlled Trials (CENTRAL), which contains the Cochrane Acute Respiratory Infections Specialised Register, Ovid MEDLINE, Embase, CINAHL, and Web of Science (from inception to 2 December 2022) with no restrictions. We searched two trial registries for ongoing trials (to 2 December 2022) and checked the reference lists of reviews and included articles for additional studies.
Selection criteria: Randomised controlled trials comparing immunoglobulins with placebo in hospitalised infants and children aged up to three years with laboratory-diagnosed RSV lower respiratory tract infection.
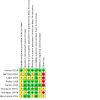
Data collection and analysis: Two review authors independently selected trials, assessed risk of bias, and extracted data. We assessed evidence certainty using GRADE.
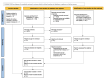
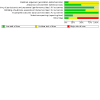
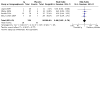
Main results: In total, we included eight trials involving 906 infants and children aged up to three years. We included one new trial in this update. The immunoglobulin preparations used in these trials included anti-RSV immunoglobulin and the monoclonal antibody preparations palivizumab and motavizumab. Five trials were conducted at single or multiple sites within a single high-income country (four in the USA, one in Qatar). Three trials included study sites in different countries. All three of these trials included study sites in one or more high-income countries (USA, Chile, New Zealand, Australia, Qatar), with two trials also including a study site in a middle-income country (Panama). Five of the eight trials were "supported" or "sponsored" by the trial drug manufacturers. The evidence is very uncertain about the effect of immunoglobulins on mortality (risk ratio (RR) 0.87, 95% confidence interval (CI) 0.14 to 5.27; 4 studies, 309 participants). There were four deaths - two amongst 98 children receiving immunoglobulins, and two amongst 98 children receiving placebo. One additional death occurred in a fourth trial, however the study group of the child was not known and the data were not included in the analysis (very low-certainty evidence). The use of immunoglobulins in infants and children admitted to hospital with RSV proven LRTI probably results in little to no difference in the length of hospitalisation (mean difference (MD) -0.13 days, 95% CI -0.37 to 0.12; 6 studies, 737 participants; moderate-certainty evidence). Immunoglobulins may result in little to no difference in the number of children who experience one or more adverse events of any severity or seriousness compared to placebo (RR 1.18, 95% CI 0.78 to 1.78; 5 studies, 340 participants; low-certainty evidence) or the number of children who experience one or more adverse events judged by study investigators to be serious in nature, compared to placebo (RR 1.08, 95% CI 0.65 to 1.79; 4 studies, 238 participants; low-certainty evidence). Certainty of evidence for secondary outcomes was low. This evidence suggests that use of immunoglobulins results in little to no difference in the need for, or duration of, mechanical ventilation and the need for, or duration of, supplemental oxygen. The use of immunoglobulins does not reduce the need for admission to the intensive care unit (ICU) and when children are admitted to the ICU results in little to no difference in the duration of ICU stay.
Authors' conclusions: We are very uncertain about the effect of immunoglobulins on mortality. We are moderately certain that use of immunoglobulins in hospitalised infants and children may result in little to no difference in the length of hospitalisation. Immunoglobulins may result in little to no difference in adverse events, the need for or duration of mechanical ventilation, supplemental oxygen, or admission to the intensive care unit, though we are less certain about this evidence and the true effect of immunoglobulins on these outcomes may differ markedly from the estimated effect observed in this review. All trials were conducted in high-income countries, and data from populations in which the rate of death from RSV infection is higher are lacking.
Copyright © 2023 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Conflict of interest statement
Sharon L Sanders: has declared that they have no conflict of interest. Sushil Agwan: has declared that they have no conflict of interest. Mohamed Hassan: has declared that they have no conflict of interest. Louis J Bont: has declared that they have the following interests: UMCU has received major funding (> EUR 100,000 per industrial partner) for investigator initiated studies from AbbVie, MedImmune, AstraZeneca, Sanofi, Janssen, Pfizer, MSD, and MeMed Diagnostics. UMCU has received major funding for the RSV GOLD study from the Bill and Melinda Gates Foundation. UMCU has received major funding as part of the public private partnership IMI‐funded RESCEU and PROMISE projects with partners GSK, Novavax, Janssen, AstraZeneca, Pfizer, and Sanofi. UMCU has received major funding by Julius Clinical for participating in clinical studies sponsored by MedImmune and Pfizer. UMCU received minor funding (EUR 1000 to 25,000 per industrial partner) for consultation and invited lectures by AbbVie, MedImmune, Ablynx, Bavaria Nordic, MabXience, GSK, Novavax, Pfizer, Moderna, AstraZeneca, MSD, Sanofi, and Janssen. Dr. Bont is the founding chairman of the ReSViNET Foundation
Figures













Update of
-
Immunoglobulin treatment for hospitalised infants and young children with respiratory syncytial virus infection.Cochrane Database Syst Rev. 2019 Aug 26;8(8):CD009417. doi: 10.1002/14651858.CD009417.pub2. Cochrane Database Syst Rev. 2019. Update in: Cochrane Database Syst Rev. 2023 Oct 23;10:CD009417. doi: 10.1002/14651858.CD009417.pub3. PMID: 31446622 Free PMC article. Updated.
References
References to studies included in this review
Alansari 2019 {published data only}
Hemming 1987 {published data only}
Lagos 2009 {published data only}
-
- Lagos R, DeVincenzo JP, Muñoz A, Hultquist M, Suzich JA, Connor EM, et al. Safety and antiviral activity of motavizumab, a respiratory syncytial virus (RSV)–specific humanized monoclonal antibody, when administered to RSV-infected children. Pediatric Infectious Disease Journal 2009;28(9):835-7. [DOI: 10.1097/INF.0b013e3181a165e4] - DOI - PubMed
Malley 1998 {published data only}
-
- Malley R, DeVincenzo J, Ramilo O, Dennehy PH, Meissner C, Gruber WC, et al. Reduction of respiratory syncytial virus (RSV) in tracheal aspirates in intubated infants by use of humanized monoclonal antibody to RSV F protein. Journal of Infectious Diseases 1998;178(6):1555-61. - PubMed
Ramilo 2014 {published data only}
-
- Ramilo O, Lagos R, Sáez-Llorens X, Suzich J, Wang CK, Jensen KM, et al, Motavizumab Study Group. Motavizumab treatment of infants hospitalized with respiratory syncytial virus infection does not decrease viral load or severity of illness. Pediatric Infectious Disease Journal 2014;33(7):703-9. [DOI: 10.1097/INF.0000000000000240] - DOI - PubMed
Rodriguez 1997a {published data only}
-
- Rodriguez WJ, Gruber WC, Welliver RC, Groothuis JR, Simoes EAF, Meissner HC, et al. Respiratory syncytial virus (RSV) immune globulin intravenous therapy for RSV lower respiratory tract infection in infants and young children at high risk for severe RSV infections. Respiratory Syncytial Virus Immune Globulin Study Group. Pediatrics 1997;99(3):454-61. - PubMed
Rodriguez 1997b {published data only}
-
- Rodriguez WJ, Gruber WC, Groothuis JR, Simoes EAF, Rosas AJ, Lepow M, et al. Respiratory syncytial virus immune globulin treatment of RSV lower respiratory tract infection in previously healthy children. Pediatrics 1997;100(6):937-42. - PubMed
Sáez‐Llorens 2004 {published data only}
-
- Sáez-Llorens X, Moreno MT, Ramilo O, Sánchez PJ, Top FH Jr, Connor EM, MEDI-493 Study Group. Safety and pharmacokinetics of palivizumab therapy in children hospitalized with respiratory syncytial virus infection. Pediatric Infectious Disease Journal 2004;23(8):707-12. [DOI: 10.1097/01.inf.0000133165.85909.08] - DOI - PubMed
References to studies excluded from this review
AAP 1998 {published data only}
-
- Committee on Infectious Diseases and Committee on Fetus and Newborn. Prevention of respiratory syncytial virus infections: indications for the use of palivizumab and update on the use of RSV-IGIV. American Academy of Pediatrics Committee on Infectious Diseases and Committee of Fetus and Newborn. Pediatrics 1998;102(5):1211-6. - PubMed
Chen 2019 {published data only}
Domachowske 2022 {published data only}
-
- Domachowske J, Madhi SA, Simoes EA, Atanosova V, Cabanas F, Furuno K, et al. Safety of nirsevimab for RSV in infants with heart or lung disease or prematurity. New England Journal of Medicine 2022;386:892-4. - PubMed
Faber 2008 {published data only}
-
- Faber TE, Kimpen JL, Bont LJ. Respiratory syncytial virus bronchiolitis: prevention and treatment. Expert Opinion on Pharmacotherapy 2008;9(14):2451-8. - PubMed
Feltes 2011 {published data only}
-
- Feltes TF, Sondheimer HM, Tulloh RM, Harris BS, Jensen KM, Losonsky GA, et al, Motavizumab Cardiac Study Group. A randomized controlled trial of motavizumab versus palivizumab for the prophylaxis of serious respiratory syncytial virus disease in children with hemodynamically significant congenital heart disease. Pediatric Research 2011;70(2):186-91. - PubMed
Fernández 2010 {published data only}
-
- Fernández P, Trenholme A, Abarca K, Griffin MP, Hultquist M, Harris B, et al, Motavizumab Study Group. A phase 2, randomized, double-blind safety and pharmacokinetic assessment of respiratory syncytial virus (RSV) prophylaxis with motavizumab and palivizumab administered in the same season. BMC Pediatrics 2010;10:38. [DOI: 10.1186/1471-2431-10-38] - DOI - PMC - PubMed
Givner 1999 {published data only}
-
- Givner LB. Monoclonal antibodies against respiratory syncytial virus. Pediatric Infectious Disease Journal 1999;18(6):541-2. - PubMed
Halsey 1997 {published data only}
-
- Halsey NA, Abramson JS, Chesney PJ, Fisher MC, Gerber MA, Gromisch DS, et al. Respiratory syncytial virus immune globulin intravenous: indications for use. Pediatrics 1997;99(4):645-50. - PubMed
Harkensee 2006 {published data only}
-
- Harkensee C, Brodlie M, Embleton ND, McKean M. Passive immunisation of preterm infants with palivizumab against RSV infection. Journal of Infection 2006;52(1):2-8. - PubMed
Helmink 2016 {published data only}
Hu 2010 {published data only}
-
- Hu J, Robinson JL. Treatment of respiratory syncytial virus with palivizumab: a systematic review. World Journal of Pediatrics 2010;6(4):296-300. - PubMed
Wegzyn 2014 {published data only}
Additional references
American Academy of Pediatrics 2014
-
- American Academy of Pediatrics Bronchiolitis Guidelines Committee. Updated guidance for palivizumab prophylaxis among infants and young children at increased risk of hospitalization for respiratory syncytial virus infection. Pediatrics 2014;134(2):415-520. - PubMed
American Academy of Pediatrics 2015
-
- American Academy of Pediatrics. Respiratory syncytial virus. In: Kimberlin DW, Brady MT, Jackson MA, Long SS, editors(s). Red Book: 2015 Report of the Committee on Infectious Diseases. 30th edition. Elk Grove Village, IL: American Academy of Pediatrics, 2015:667-76.
American Academy of Pediatrics 2018
-
- American Academy of Pediatrics. Respiratory syncytial virus. In: Kimberlin DW, Brady MT, Jackson MA, Long SS, editors(s). Red Book: 2018 Report of the Committee on Infectious Diseases. 31st edition. Itasca, IL: American Academy of Pediatrics, 2018:682-92.
Broadbent 2015
-
- Broadbent L, Groves H, Shields MD, Power UF. Respiratory syncytial virus, an ongoing medical dilemma: an expert commentary on respiratory syncytial virus prophylactic and therapeutic pharmaceuticals currently in clinical trials. Influenza and Other Respiratory Viruses 2015;9(4):169-78. - PMC - PubMed
Feltes 2003
-
- Feltes TF, Cablka AK, Meissner HC, Piazza FM, Carlin DA, Top FH Jr, et al. Palivizumab prophylaxis reduces hospitalization due to respiratory syncytial virus in young children with hemodynamically significant congenital heart disease. Journal of Pediatrics 2003;143(4):532-40. - PubMed
Fernandes 2013
Gadomski 2014
Geerdink 2015
GRADEpro GDT [Computer program]
-
- GRADEpro GDT. Version accessed April 2023. Hamilton (ON): McMaster University (developed by Evidence Prime). Available from gradepro.org.
Greenough 2001
Griffiths 2017
Groothuis 1993
-
- Groothuis JR, Simoes EA, Levin MJ, Hall CB, Long CE, Rodriguez WJ, et al. Prophylactic administration of respiratory syncytial virus immune globulin to high-risk infants and young children. The Respiratory Syncytial Virus Immune Globulin Study Group. New England Journal of Medicine 1993;329(21):1524-30. - PubMed
Hammitt 2022
-
- Hammitt LL, Dagan R, Yuan Y, Baca Cots M, Bosheva M, Madhi SA. Nirsevimab for prevention of RSV in healthy late-preterm and term infants. New England Journal of Medicine 2022;386(9):837-46. - PubMed
Hartling 2011
Higgins 2022
-
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 6.3 (updated February 2022). Cochrane, 2022. Available from training.cochrane.org/handbook.
Hozo 2005
Jolles 2005
Kampmann 2023
-
- Kampmann B, Madhi SA, Munjal I, Simeos EA, Pahud BA, Llapur C. Bivalent perfusion F vaccine in pregnancy to prevent RSV illness in infants. New England Journal of Medicine 2023;388(16):1451-64. - PubMed
Langley 1997
-
- Langley JM, Wang EE, Law BJ, Stephens D, Boucher FD, Dobson S, et al. Economic evaluation of respiratory syncytial virus infection in Canadian children: a Pediatric Investigators Collaborative Network on Infections in Canada (PICNIC) study. Journal of Pediatrics 1997;131:113-7. - PubMed
Lefebvre 2011
-
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1/.
Li 2023
Marshall 2018
Mayo Clinic 2017
-
- Mayo Clinic. Respiratory syncytial virus. www.mayoclinic.org/diseases-conditions/respiratory-syncytial-virus/sympt... (accessed prior to 9 January 2019).
Mejías 2005
Nair 2010
Oray‐Schrom 2003
-
- Oray-Schrom P, Phoenix C, St Martin D, Amoateng-Adjepong Y. Sepsis workup in febrile infants 0-90 days of age with respiratory syncytial virus infection. Pediatric Emergency Care 2003;19(5):314-9. - PubMed
Paramore 2004
-
- Paramore LC, Ciuryla V, Ciesla G, Liu L. Economic impact of respiratory syncytial virus-related illness in the US: an analysis of national databases. Pharmacoeconomics 2004;22:275-84. - PubMed
PREVENT 1997
-
- PREVENT Study Group. Reduction of RSV hospitalization among premature infants and infants with bronchopulmonary dysplasia using respiratory syncytial virus immune globulin prophylaxis. Pediatrics 1997;99(1):93-9. - PubMed
Ralston 2009
-
- Ralston S, Hill V. Incidence of apnea in infants hospitalized with respiratory syncytial virus bronchiolitis: a systematic review. Journal of Pediatrics 2009;155(5):728-33. - PubMed
Resch 2017
RevMan Web 2022 [Computer program]
-
- Review Manager Web (RevMan Web). Version 4.12.0. Copenhagen: The Cochrane Collaboration, 2022. Available at revman.cochrane.org.
Roche 2003
-
- Roche P, Lambert S, Spencer J. Surveillance of viral pathogens in Australia: respiratory syncytial virus. Communicable Diseases Intelligence 2003;27(1):117-22. - PubMed
Rodriguez 1997
-
- Rodriguez WJ, Gruber WC, Groothuis JR, Simoes EA, Rosas AJ, Lepow M, et al. Respiratory syncytial virus immune globulin treatment of RSV lower respiratory tract infection in previously healthy children. Pediatrics 2007;100(6):937-42. - PubMed
Santesso 2020
-
- Santesso N, Genton C, Dahm P, Garner P, Akl EA, Alper B, et al. GRADE guidelines 26: informative statements to communicate the findings of systematic reviews of interventions. Journal of Clinical Epidemiology 2020;119:126-35. - PubMed
Schünemann 2013
-
- Schünemann H, Brożek J, Guyatt G, Oxman A, editor(s). Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach (updated October 2013). GRADE Working Group, 2013. Available from gdt.guidelinedevelopment.org/app/handbook/handbook.html.
Shi 2017
-
- Shi T, McAllister DA, O'Brien KL, Simoes EA, Madhi SA, Gessner BD, et al, RSV Global Epidemiology Network. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in young children in 2015: a systematic review and modelling study. Lancet 2017;390(10098):946-58. - PMC - PubMed
Synagis 2017
-
- Synagis. Highlights of prescribing information. www.azpicentral.com/synagis/synagis.pdf (accessed prior to 5 December 2018).
Turner 2014
Wang 2011
Zhang 2017
References to other published versions of this review
Fuller 2004
Fuller 2006
Kantharajah 2011
-
- Kantharajah M, Zaman FD, Samra D, Gunturu MN, Del Mar CB, Driel ML. Immunoglobulin treatment for respiratory syncytial virus infection. Cochrane Database of Systematic Reviews 2011, Issue 11. Art. No: CD009417. [DOI: 10.1002/14651858.CD009417] - DOI
Sanders 2019
Tan 1998
-
- Tan D, Wang E, Ohlsson A. Immunoglobulin for treatment of respiratory syncytial virus. Cochrane Database of Systematic Reviews 1998, Issue 1. Art. No: CD000981. [DOI: 10.1002/14651858.CD000981] - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

