Synbiotics, prebiotics and probiotics for people with chronic kidney disease
- PMID: 37870148
- PMCID: PMC10591284
- DOI: 10.1002/14651858.CD013631.pub2
Synbiotics, prebiotics and probiotics for people with chronic kidney disease
Abstract
Background: Chronic kidney disease (CKD) is a major public health problem affecting 13% of the global population. Prior research has indicated that CKD is associated with gut dysbiosis. Gut dysbiosis may lead to the development and/or progression of CKD, which in turn may in turn lead to gut dysbiosis as a result of uraemic toxins, intestinal wall oedema, metabolic acidosis, prolonged intestinal transit times, polypharmacy (frequent antibiotic exposures) and dietary restrictions used to treat CKD. Interventions such as synbiotics, prebiotics, and probiotics may improve the balance of the gut flora by altering intestinal pH, improving gut microbiota balance and enhancing gut barrier function (i.e. reducing gut permeability).
Objectives: This review aimed to evaluate the benefits and harms of synbiotics, prebiotics, and probiotics for people with CKD.
Search methods: We searched the Cochrane Kidney and Transplant Register of Studies up to 9 October 2023 through contact with the Information Specialist using search terms relevant to this review. Studies in the Register are identified through searches of CENTRAL, MEDLINE, and EMBASE, conference proceedings, the International Clinical Trials Registry Platform (ICTRP) Search Portal and ClinicalTrials.gov.
Selection criteria: We included randomised controlled trials (RCTs) measuring and reporting the effects of synbiotics, prebiotics, or probiotics in any combination and any formulation given to people with CKD (CKD stages 1 to 5, including dialysis and kidney transplant). Two authors independently assessed the retrieved titles and abstracts and, where necessary, the full text to determine which satisfied the inclusion criteria.
Data collection and analysis: Data extraction was independently carried out by two authors using a standard data extraction form. Summary estimates of effect were obtained using a random-effects model, and results were expressed as risk ratios (RR) and their 95% confidence intervals (CI) for dichotomous outcomes, and mean difference (MD) or standardised mean difference (SMD) and 95% CI for continuous outcomes. The methodological quality of the included studies was assessed using the Cochrane risk of bias tool. Data entry was carried out by one author and cross-checked by another. Confidence in the evidence was assessed using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach.
Main results: Forty-five studies (2266 randomised participants) were included in this review. Study participants were adults (two studies in children) with CKD ranging from stages 1 to 5, with patients receiving and not receiving dialysis, of whom half also had diabetes and hypertension. No studies investigated the same synbiotic, prebiotic or probiotic of similar strains, doses, or frequencies. Most studies were judged to be low risk for selection bias, performance bias and reporting bias, unclear risk for detection bias and for control of confounding factors, and high risk for attrition and other biases. Compared to prebiotics, it is uncertain whether synbiotics improve estimated glomerular filtration rate (eGFR) at four weeks (1 study, 34 participants: MD -3.80 mL/min/1.73 m², 95% CI -17.98 to 10.38), indoxyl sulfate at four weeks (1 study, 42 participants: MD 128.30 ng/mL, 95% CI -242.77 to 499.37), change in gastrointestinal (GI) upset (borborymgi) at four weeks (1 study, 34 participants: RR 15.26, 95% CI 0.99 to 236.23), or change in GI upset (Gastrointestinal Symptom Rating Scale) at 12 months (1 study, 56 participants: MD 0.00, 95% CI -0.27 to 0.27), because the certainty of the evidence was very low. Compared to certain strains of prebiotics, it is uncertain whether a different strain of prebiotics improves eGFR at 12 weeks (1 study, 50 participants: MD 0.00 mL/min, 95% CI -1.73 to 1.73), indoxyl sulfate at six weeks (2 studies, 64 participants: MD -0.20 μg/mL, 95% CI -1.01 to 0.61; I² = 0%) or change in any GI upset, intolerance or microbiota composition, because the certainty of the evidence was very low. Compared to certain strains of probiotics, it is uncertain whether a different strain of probiotic improves eGFR at eight weeks (1 study, 30 participants: MD -0.64 mL/min, 95% CI -9.51 to 8.23; very low certainty evidence). Compared to placebo or no treatment, it is uncertain whether synbiotics improve eGFR at six or 12 weeks (2 studies, 98 participants: MD 1.42 mL/min, 95% CI 0.65 to 2.2) or change in any GI upset or intolerance at 12 weeks because the certainty of the evidence was very low. Compared to placebo or no treatment, it is uncertain whether prebiotics improves indoxyl sulfate at eight weeks (2 studies, 75 participants: SMD -0.14 mg/L, 95% CI -0.60 to 0.31; very low certainty evidence) or microbiota composition because the certainty of the evidence is very low. Compared to placebo or no treatment, it is uncertain whether probiotics improve eGFR at eight, 12 or 15 weeks (3 studies, 128 participants: MD 2.73 mL/min, 95% CI -2.28 to 7.75; I² = 78%), proteinuria at 12 or 24 weeks (1 study, 60 participants: MD -15.60 mg/dL, 95% CI -34.30 to 3.10), indoxyl sulfate at 12 or 24 weeks (2 studies, 83 participants: MD -4.42 mg/dL, 95% CI -9.83 to 1.35; I² = 0%), or any change in GI upset or intolerance because the certainty of the evidence was very low. Probiotics may have little or no effect on albuminuria at 12 or 24 weeks compared to placebo or no treatment (4 studies, 193 participants: MD 0.02 g/dL, 95% CI -0.08 to 0.13; I² = 0%; low certainty evidence). For all comparisons, adverse events were poorly reported and were minimal (flatulence, nausea, diarrhoea, abdominal pain) and non-serious, and withdrawals were not related to the study treatment.
Authors' conclusions: We found very few studies that adequately test biotic supplementation as alternative treatments for improving kidney function, GI symptoms, dialysis outcomes, allograft function, patient-reported outcomes, CVD, cancer, reducing uraemic toxins, and adverse effects. We are not certain whether synbiotics, prebiotics, or probiotics are more or less effective compared to one another, antibiotics, or standard care for improving patient outcomes in people with CKD. Adverse events were uncommon and mild.
Trial registration: ClinicalTrials.gov NCT02706808 NCT02008331 NCT02704884 NCT01450709 NCT02141815 NCT02364869 NCT00760162 NCT01186276 NCT01391468 NCT01752803 NCT01735591 NCT04204005 NCT03689569 NCT03770611 NCT03789708 NCT03924089.
Copyright © 2023 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Conflict of interest statement
TC: none known
RK: none known
SC: none known
JC: none known
Carmel Hawley has received fees paid to her institution from Janssen and GlaxoSmithKline; Advisory Board fees paid to her from Otsuka; Research Grants to her institution from Otsuka, Shire, Fresenius, and Baxter; none of these are related to the current study. In addition, she has received grants paid to her institution from the Polycystic Kidney Disease foundation of Australia and from Otsuka for work that is related to the current study
MH: none known
David Johnson has received consultancy fees, research grants, speaker's honoraria and travel sponsorships from Baxter Healthcare and Fresenius Medical Care. He has received consultancy fees from AstraZeneca and AWAK, and travel sponsorships from Amgen
ATP: none known
AT: none known
GW: none known
Figures
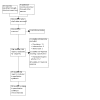
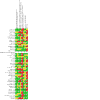












































































Update of
References
References to studies included in this review
Abbasi 2017 {published data only}
-
- Abbasi B, Ghiasvand R, Mirlohi M. Kidney function improvement by soy milk containing lactobacillus plantarum A7 in type 2 diabetic patients with nephropathy: a double-blinded randomized controlled trial. Iranian Journal of Kidney Diseases 2017;11(1):36-43. [MEDLINE: ] - PubMed
-
- Abbasi B, Mirlohi M, Daniali M, Ghiasvand R. Effects of probiotic soy milk on lipid panel in type 2 diabetic patients with nephropathy: a double-blind randomized clinical trial. Progress in Nutrition 2018;20:70-8. [EMBASE: 2001649336]
Biruete 2017 {published data only}
-
- Biruete A, Kistler B, Allen J, Bauer L, Fahey G, Swanson K, et al. Effect of inulin supplementation on mineral metabolism and short-chain fatty acid excretion in hemodialysis patients [abstract no: MP434]. Nephrology Dialysis Transplantation 2017;32(Suppl 3):iii588-9. [EMBASE: 617289888]
Bliss 1992 {published data only}
-
- Bliss DZ, Stein TP, Schleifer CR, Settle RG. Supplementation with gum arabic fiber increases fecal nitrogen excretion and lowers serum urea nitrogen concentration in chronic renal failure patients consuming a low-protein diet. American Journal of Clinical Nutrition 1996;63(3):392-8. [MEDLINE: ] - PubMed
-
- Bliss DZ. Effect of a gum Arabic supplement on the nitrogen excretion and serum urea nitrogen concentration of chronic renal failure patients on a low protein diet [PhD thesis]. University of Pennsylvania 1992:1-206. [CINAHL: 109870911]
Borges 2018 {published data only}
-
- Borges NA, Carmo FL, Stockler-Pinto MB, Brito JS, Dolenga CJ, Ferreira DC, et al. Probiotic supplementation in chronic kidney disease: a double-blind, randomized, placebo-controlled trial. Journal of Renal Nutrition 2018;28(1):28-36. [MEDLINE: ] - PubMed
-
- Borges NA, Stenvinkel P, Bergman P, Qureshi AR, Lindholm B, Moraes C, et al. Effects of probiotic supplementation on trimethylamine-N-oxide plasma levels in hemodialysis patients: a pilot study. Probiotics & Antimicrobial Proteins 2019;11(2):648-54. [MEDLINE: ] - PubMed
-
- Mafra D, Borges NA, Moraes C, Stockler-Pinto MB, Bergman P, Stenvinkel P. Effects of probiotic supplementation on trimethylamine-N-oxide plasma levels in chronic kidney disease patients [abstract no: TH-PO764]. Journal of the American Society of Nephrology 2016;27(Abstract Suppl):270A. [EMBASE: 641135082]
-
- Mafra D, Borges NA, Nakau L, Dolenga C, Bergman P, Stenvinkel P. Effects of probiotic supplementation on uremic toxins levels in non-dialysis CKD patients [abstract no: MP431]. Nephrology Dialysis Transplantation 2017;32(Suppl 3):iii587-8. [EMBASE: 617289825]
-
- Mafra D, Borges NA, Stockler-Pinto MB, Fouque D, Barros AF. Does a probiotic supplementation alter the indoxyl sulfate levels in non-dialysis chronic kidney disease patients? A randomized placebo-controlled clinical trial [abstract no: FR-PO862]. Journal of the American Society of Nephrology 2015;26(Abstract Suppl):563A. [EMBASE: 641102246]
Cosola 2021 {published data only}
-
- Cosola C, Rocchetti MT, di Bari I, Acquaviva PM, Maranzano V, Corciulo S, et al. An innovative synbiotic formulation decreases free serum indoxyl sulfate, small intestine permeability and ameliorates gastrointestinal symptoms in a randomized pilot trial in stage IIIb-IV CKD patients. Toxins 2021;13(5):334. [MEDLINE: ] - PMC - PubMed
de Andrade 2021 {published data only}
-
- Andrade LS, Sarda FA, Pereira NB, Teixeira RR, Rodrigues SD, Lima JD, et al. Effect of unripe banana flour on gut-derived uremic toxins in individuals undergoing peritoneal dialysis: a randomized, double-blind, placebo-controlled, crossover trial. Nutrients 2021;13(2):646. [MEDLINE: ] - PMC - PubMed
de Araujo 2022 {published data only}
-
- De Araujo EM, Meneses GC, Martins AM, De Francesco Daher E, Da Silva Junior GB. Effect of probiotic supplementation on endothelial injury and inflammation biomarkers among patients with chronic kidney diseases on hemodialysis [abstract no: MO584]. Nephrology Dialysis Transplantation 2022;37(Suppl 3):i429. [DOI: 10.1093/ndt/gfac074.029] - DOI
Dehghani 2016 {published data only}
-
- Dehghani H, Heidari F, Mozaffari-Khosravi H, Nouri-Majelan N, Dehghani A. Synbiotic supplementations for azotemia in patients with chronic kidney disease: a randomized controlled trial. Iranian Journal of Kidney Diseases 2016;10(6):351-7. [MEDLINE: ] - PubMed
Ebrahim 2022 {published data only}
-
- Ebrahim Z, Proost S, Tito RY, Raes J, Glorieux G, Moosa MR, et al. The effect of s-glucan prebiotic on kidney function, uremic toxins and gut microbiome in stage 3 to 5 chronic kidney disease (CKD) predialysis participants: a randomized controlled trial. Nutrients 2022;14(4):805. [MEDLINE: ] - PMC - PubMed
Eidi 2018 {published data only}
-
- Eidi F, Poor-Reza GF, Ostadrahimi A, Dalili N, Samadian F, Barzegari A. Effect of Lactobacillus rhamnosus on serum uremic toxins (phenol and P-cresol) in hemodialysis patients: A double blind randomized clinical trial. Clinical Nutrition ESPEN 2018;28:158-64. [MEDLINE: ] - PubMed
-
- Gholi FP, Dalili N, Eidi F, Ostadrahimi A. Effect of lactobacillus rhamnosus on serum uremic toxins in hemodialysis patients [abstract no: SP705]. Nephrology Dialysis Transplantation 2017;32(Suppl 3):iii375. [EMBASE: 617290088]
Elamin 2017 {published data only}
Esgalhado 2018 {published data only}
-
- Azevedo R, Esgalhado M, Kemp JA, Regis B, Cardozo LF, Nakao LS, et al. Resistant starch supplementation effects on plasma indole 3-acetic acid and aryl hydrocarbon receptor mRNA expression in hemodialysis patients: Randomized, double blind and controlled clinical trial [Efeitos da suplementacao de amido resistente no acido indol-3-acetico plasmatico e na expressao do mRNA do receptor aril-hidrocarboneto em pacientesemhemodialise:ensaioclinicorandomizado,duplo-cegoecontrolado]. Jornal Brasileiro de Nefrologia 2020;42(3):273-9. [MEDLINE: ] - PMC - PubMed
-
- Esgalhado M, Kemp JA, Azevedo R, Paiva BR, Stockler-Pinto MB, Dolenga CJ, et al. Could resistant starch supplementation improve inflammatory and oxidative stress biomarkers and uremic toxins levels in hemodialysis patients? A pilot randomized controlled trial. Food & Function 2018;9(12):6508-16. [MEDLINE: ] - PubMed
-
- Esgalhado M, Kemp JA, Paiva BR, Brito JS, Cardozo LF, Azevedo R, et al. Resistant starch type-2 enriched cookies modulate uremic toxins and inflammation in hemodialysis patients: a randomized, double-blind, crossover and placebo-controlled trial. Food & Function 2020;11(3):2617-25. [MEDLINE: ] - PubMed
-
- Kemp JA, Dos Santos HF, Jesus HE, Esgalhado M, Paiva BR, Azevedo R, et al. Resistant starch type-2 supplementation does not decrease trimethylamine n-oxide (TMAO) plasma level in hemodialysis patients. Journal of the American Nutrition Association 2022;41(8):788-95. [MEDLINE: ] - PubMed
-
- Kemp JA, Regis de Paiva B, Fragoso Dos Santos H, Emiliano de Jesus H, Craven H, Z Ijaz U, et al. The impact of enriched resistant starch type-2 cookies on the gut microbiome in hemodialysis patients: a randomized controlled trial. Molecular Nutrition & Food Research 2021;65(19):e2100374. [MEDLINE: ] - PubMed
Guida 2014 {published data only}
-
- Guida B, Germano R, Trio R, Russo D, Memoli B, Grumetto L, et al. Effect of short-term synbiotic treatment on plasma p-cresol levels in patients with chronic renal failure: a randomized clinical trial. Nutrition Metabolism & Cardiovascular Diseases 2014;24(9):1043-9. [MEDLINE: ] - PubMed
Guida 2017 {published data only}
-
- Guida B, Cataldi M, Memoli A, Trio R, di Maro M, Grumetto L, et al. Effect of a short-course treatment with synbiotics on plasma p-cresol concentration in kidney transplant recipients. Journal of the American College of Nutrition 2017;36(7):586-91. [PMID: ] - PubMed
Haghighat 2019 {published data only}
-
- Haghighat N, Mohammadshahi M, Shayanpour S, Haghighizadeh MH, Rahmdel S, Rajaei M. The effect of synbiotic and probiotic supplementation on mental health parameters in patients undergoing hemodialysis: A double-blind, randomized, placebo-controlled trial. Indian Journal of Nephrology 2021;31(2):149-56. [EMBASE: 634933481] - PMC - PubMed
-
- Haghighat N, Mohammadshahi M, Shayanpour S, Haghighizadeh MH. Effect of synbiotic and probiotic supplementation on serum levels of endothelial cell adhesion molecules in hemodialysis patients: a randomized control study. Probiotics & Antimicrobial Proteins 2019;11(4):1210-8. [MEDLINE: ] - PubMed
-
- Haghighat N, Mohammadshahi M, Shayanpour S, Haghighizadeh MH. Effects of synbiotics and probiotics supplementation on serum levels of endotoxin, heat shock protein 70 antibodies and inflammatory markers in hemodialysis patients: a randomized double-blinded controlled trial. Probiotics & Antimicrobial Proteins 2020;12(1):144-51. [MEDLINE: ] - PubMed
-
- Haghighat N, Rajabi S, Mohammadshahi M. Effect of synbiotic and probiotic supplementation on serum brain-derived neurotrophic factor level, depression and anxiety symptoms in hemodialysis patients: a randomized, double-blinded, clinical trial. Nutritional Neuroscience 2019;24(6):490-9. [MEDLINE: ] - PubMed
-
- Haghighat N, Rajabi S, Mohammadshahi M. Effect of synbiotic and probiotic supplementation on serum brain-derived neurotrophic factor level, depression and anxiety symptoms in hemodialysis patients: a randomized, double-blinded, clinical trial. Nutritional Neuroscience 2021;24(6):490-9. [MEDLINE: ] - PubMed
He 2022 {published data only}
-
- He S, Xiong Q, Tian C, Li L, Zhao J, Lin X, et al. Inulin-type prebiotics reduce serum uric acid levels via gut microbiota modulation: a randomized, controlled crossover trial in peritoneal dialysis patients. European Journal of Nutrition 2022;61(2):665-77. [MEDLINE: ] - PubMed
Khosroshahi 2018 {published data only}
-
- Khosroshahi HT, Abedi B, Ghojazadeh M, Samadi A, Jouyban A. Effects of fermentable high fiber diet supplementation on gut derived and conventional nitrogenous product in patients on maintenance hemodialysis: a randomized controlled trial. Nutrition & Metabolism 2019;16:18. [MEDLINE: ] - PMC - PubMed
-
- Laffin MR, Tayebi Khosroshahi H, Park H, Laffin LJ, Madsen K, Kafil HS, et al. Amylose resistant starch (HAM-RS2) supplementation increases the proportion of Faecalibacterium bacteria in end-stage renal disease patients: microbial analysis from a randomized placebo-controlled trial. Hemodialysis International 2019;23(3):343-7. [MEDLINE: ] - PubMed
-
- Tayebi Khosroshahi H, Vaziri ND, Abedi B, Asl BH, Ghojazadeh M, Jing W, et al. Effect of high amylose resistant starch (HAM-RS2) supplementation on biomarkers of inflammation and oxidative stress in hemodialysis patients: a randomized clinical trial. Hemodialysis International 2018;22(4):492-500. [MEDLINE: ] - PubMed
Kooshki 2019 {published data only}
-
- Kooshki A, Tofighiyan T, Miri M. A synbiotic supplement for inflammation and oxidative stress and lipid abnormalities in hemodialysis patients. Hemodialysis International 2019;23(2):254-60. [MEDLINE: ] - PubMed
Li 2020 {published data only}
-
- Li L, Xiong Q, Zhao J, Lin X, He S, Wu N, et al. Inulin-type fructan intervention restricts the increase in gut microbiome-generated indole in patients with peritoneal dialysis: a randomized crossover study [Erratum in: Am J Clin Nutr. 2022 Jun 7;115(6):1659]. American Journal of Clinical Nutrition 2020;111(5):1087-99. [MEDLINE: ] - PubMed
Lim 2021 {published data only}
-
- Lim PS, Wang HF, Lee MC, Chiu LS, Wu MY, Chang WC, et al. The efficacy of lactobacillus-containing probiotic supplementation in hemodialysis patients: a randomized, double-blind, placebo-controlled trial. Journal of Renal Nutrition 2021;31(2):189-98. [MEDLINE: ] - PubMed
Liu 2020 {published data only}
-
- Liu S, Liu H, Chen L, Liang SS, Shi K, Meng W, et al. Effect of probiotics on the intestinal microbiota of hemodialysis patients: a randomized trial. European Journal of Nutrition 2020;59(8):3755-66. [MEDLINE: ] - PubMed
Lopes 2018 {published data only}
-
- Lopes RC, Theodoro JM, da Silva BP, Queiroz VA, Castro Moreira ME, Mantovani HC, et al. Synbiotic meal decreases uremic toxins in hemodialysis individuals: a placebo-controlled trial. Food Research International 2019;116:241-8. [MEDLINE: ] - PubMed
-
- Lopes RC, Lima SL, da Silva BP, Toledo RC, Moreira ME, Anunciacao PC, et al. Evaluation of the health benefits of consumption of extruded tannin sorghum with unfermented probiotic milk in individuals with chronic kidney disease. Food Research International 2018;107:629-38. [MEDLINE: ] - PubMed
Lydia 2022 {published data only}
Mafi 2018 {published data only}
-
- Mafi A, Namazi G, Soleimani A, Bahmani F, Aghadavod E, Asemi Z. Metabolic and genetic response to probiotics supplementation in patients with diabetic nephropathy: a randomized, double-blind, placebo-controlled trial [Expression of Concern in: Food Funct. 2022 Apr 4;13(7):4229]. Food & Function 2018;9(9):4763-70. [MEDLINE: ] - PubMed
Mazruei Arani 2019 {published data only}
-
- Mazruei Arani N, Emam-Djomeh Z, Tavakolipour H, Sharafati-Chaleshtori R, Soleimani A, Asemi Z. The effects of probiotic honey consumption on metabolic status in patients with diabetic nephropathy: a randomized, double-blind, controlled trial. Probiotics & Antimicrobial Proteins 2019;11(4):1195-201. [MEDLINE: ] - PubMed
Meng 2019 {published data only}
-
- Meng Y, Bai H, Yu Q, Yan J, Zhao L, Wang S, et al. High-resistant starch, low-protein flour intervention on patients with early type 2 diabetic nephropathy: a randomized trial. Journal of Renal Nutrition 2019;29(5):386-93. [MEDLINE: ] - PubMed
Miraghajani 2017 {published data only}
-
- Miraghajani M, Zaghian N, Dehkohneh A, Mirlohi M, Ghiasvand R. Probiotic soy milk consumption and renal function among type 2 diabetic patients with nephropathy: a randomized controlled clinical trial. Probiotics & Antimicrobial Proteins 2019;11(1):124-32. [MEDLINE: ] - PubMed
-
- Miraghajani M, Zaghian N, Mirlohi M, Feizi A, Ghiasvand R. Impact of probiotic soy milk consumption on oxidative stress among type 2 diabetic kidney disease patients: a randomized controlled clinical trial. Journal of Renal Nutrition 2017;27(5):317-24. [MEDLINE: ] - PubMed
Miranda Alatriste 2014 {published data only}
-
- Miranda Alatriste PV, Urbina AR, Gomez Espinosa CO, Espinosa Cuevas ML. Effect of probiotics on human blood urea levels in patients with chronic renal failure. Nutricion Hospitalaria 2014;29(3):582-90. [MEDLINE: ] - PubMed
Mirzaeian 2020 {published data only}
-
- Mirzaeian S, Saraf-Bank S, Entezari MH, Hekmatdoost A, Feizi A, Atapour A. Effects of synbiotic supplementation on microbiota-derived protein-bound uremic toxins, systemic inflammation, and biochemical parameters in patients on hemodialysis: A double-blind, placebo-controlled, randomized clinical trial. Nutrition 2020;73:110713. [MEDLINE: ] - PubMed
Natarajan 2014 {published data only}
Pan 2021 {published data only}
-
- Pan Y, Yang L, Dai B, Lin B, Lin S, Lin E. Effects of probiotics on malnutrition and health-related quality of life in patients undergoing peritoneal dialysis: a randomized controlled trial. Journal of Renal Nutrition 2021;31(2):199-205. [MEDLINE: ] - PubMed
Poesen 2016 {published data only}
-
- Poesen R, Evenepoel P, Loor H, Delcour JA, Courtin CM, Kuypers D, et al. The influence of prebiotic arabinoxylan oligosaccharides on microbiota derived uremic retention solutes in patients with chronic kidney disease: a randomized controlled trial. PLoS ONE {Electronic Resource] 2016;11(4):e0153893. [MEDLINE: ] - PMC - PubMed
PREBIOTIC 2022 {published data only}
-
- Chan S, Hawley CM, Pascoe EM, Cao C, Campbell KL, Campbell SB, et al. PREBIOTIC: a study protocol of a randomised controlled trial to assess prebiotic supplementation in kidney transplant recipients for preventing infections and gastrointestinal upset — a feasibility study. Pilot & Feasibility Studies 2023;9(1):11. [DOI: 10.1186/s40814-023-01236-y] - DOI - PMC - PubMed
-
- Chan S, Hawley CM, Pascoe EM, Cao C, Campbell SB, Campbell KL, et al. Prebiotic supplementation in kidney transplant recipients for preventing infections and gastrointestinal upset: a randomized controlled feasibility study. Journal of Renal Nutrition 2022;32(6):718-25. [MEDLINE: ] - PubMed
-
- Chan S, Hawley CM, Pascoe EM, Cao CZ, Campbell SB, Campbell KL, et al. Prebiotic supplementation in kidney transplant recipients for preventing infections and gastrointestinal upset: a randomized controlled feasibility study. American Journal of Transplantation 2022;22:783. [DOI: 10.1111/ajt.17073] - DOI - PubMed
ProbiotiCKD 2019 {published data only}
-
- Simeoni M, Cianfrone P, Capria M, Deodato F, Citraro M, Cerantonio A, et al. ProbiotiCKD: A monocentric, randomized, openlabel, placebo-controlled study to reveal and correct gut dysbiosis in early CKD stages [abstract no: SP367]. Nephrology Dialysis Transplantation 2018;33(Suppl 1):i469-70. [EMBASE: 622605912]
-
- Simeoni M, Citraro ML, Cerantonio A, Deodato F, Provenzano M, Cianfrone P, et al. An open-label, randomized, placebo-controlled study on the effectiveness of a novel probiotics administration protocol (ProbiotiCKD) in patients with mild renal insufficiency (stage 3a of CKD) [Erratum in: Eur J Nutr. 2018 58(5):2157]. European Journal of Nutrition 2019;58(5):2145-56. [MEDLINE: ] - PMC - PubMed
-
- Simeoni M, Citraro ML, Cerantonio A, Deodato F, Provenzano M, Cianfrone P, et al. Correction to: An open-label, randomized, placebo-controlled study on the effectiveness of a novel probiotics administration protocol (ProbiotiCKD) in patients with mild renal insufficiency (stage 3a of CKD) (European Journal of Nutrition, (2019), 58, 5, (2145-2156), 10.1007/s00394-018-1785-z). European Journal of Nutrition 2019;58(5):2157. [MEDLINE: ] - PMC - PubMed
Ramos 2019 {published data only}
-
- Armani R, Ishikawa C, Hong V, Bortolotto LA, Cassiolatto JL, Klassen A, et al. Effect of frutooligosaccharide on endothelial function in CKD patients: a randomized controlled trial [abstract no: SAT-206]. Kidney International Reports 2019;4(7 Suppl):S93. [EMBASE: 2002179674]
-
- Armani RG, Carvalho AB, Ramos CI, Hong V, Bortolotto LA, Cassiolato JL, et al. Effect of fructooligosaccharide on endothelial function in CKD patients: a randomized controlled trial. Nephrology Dialysis Transplantation 2021;37(1):85-91. [MEDLINE: ] - PubMed
-
- Armani RG, Ramos CI, Cuppari L, Canziani ME. Effect of fructooligosaccharide on endothelium function in CKD patients: a randomized controlled trial [abstract no: TH-PO1138]. Journal of the American Society of Nephrology 2018;29(Abstract Suppl):417. [EMBASE: 633736090]
-
- Ramos CI, Armani RG, Canziani MEF, Dalboni MA, Dolenga CJR, Nakao LS, et al. Effect of prebiotic (fructooligosaccharide) on uremic toxins of chronic kidney disease patients: a randomized controlled trial. Nephrology Dialysis Transplantation 2019;34(11):1876-84. [MEDLINE: ] - PubMed
-
- Ramos CI, Armani RG, Nakao LS, Canziani MEF, Campbell KL, Cuppari L. Effect of fructooligosaccharide on microbiota-derived uremic toxins in predialysis patients: a randomized controlled trial [abstract no: SA-PO161]. Journal of the American Society of Nephrology 2017;28(Abstract Suppl):720. [EMBASE: 633702127]
Ranganathan 2009 {published data only}
-
- Ranganathan N, Friedman EA, Tam P, Rao V, Ranganathan P, Dheer R. Probiotic dietary supplementation in patients with stage 3 and 4 chronic kidney disease: a 6-month pilot scale trial in Canada. Current Medical Research & Opinion 2009;25(8):1919-30. [MEDLINE: ] - PubMed
-
- Ranganathan N, Friedman EA, Tam PY, Rao V, Ranganathan PL. Dietary supplementation with a probiotic formulation (Kibow biotics) on CKD III and IV patients- a short term pilot scale study in Canada [abstract no: SA-PO2755]. Journal of the American Society of Nephrology 2009;20(Abstract Suppl):741A.
-
- Ranganathan N, Ranganathan P, Friedman EA, Joseph A, Delano B, Goldfarb DS, et al. Pilot study of probiotic dietary supplementation for promoting healthy kidney function in patients with chronic kidney disease. Advances in Therapy 2010;27(9):634-47. [MEDLINE: ] - PubMed
Shariaty 2017 {published data only}
Sirich 2014 {published data only}
Soleimani 2017 {published data only}
-
- Soleimani A, Zarrati Mojarrad M, Bahmani F, Taghizadeh M, Ramezani M, Tajabadi-Ebrahimi M, et al. Probiotic supplementation in diabetic hemodialysis patients has beneficial metabolic effects. Kidney International 2017;91(2):435-42. [MEDLINE: ] - PubMed
Soleimani 2019 {published data only}
-
- Soleimani A, Motamedzadeh A, Zarrati Mojarrad M, Bahmani F, Amirani E, Ostadmohammadi V, et al. The effects of synbiotic supplementation on metabolic status in diabetic patients undergoing hemodialysis: a randomized, double-blinded, placebo-controlled trial. Probiotics & Antimicrobial Proteins 2019;11(4):1248-56. [MEDLINE: ] - PubMed
SYNERGY 2014 {published data only}
-
- Rossi M, Johnson D, Pascoe E, Coombes J, Forbes J, Szeto CC, et al. Synbiotic therapy in pre-dialysis: a randomised controlled trial [abstract no: SAO029]. Nephrology Dialysis Transplantation 2015;30(Suppl 3):iii38. [EMBASE: 72206363]
-
- Rossi M, Johnson DW, Xu H, Carrero JJ, Pascoe E, French C, et al. Dietary protein-fiber ratio associates with circulating levels of indoxyl sulfate and p-cresyl sulfate in chronic kidney disease patients. Nutrition Metabolism & Cardiovascular Diseases 2015;25(9):860-5. [MEDLINE: ] - PubMed
SYNERGY II 2021 {published data only}ACTRN12617000324314
Viramontes‐Horner 2015 {published data only}
-
- Viramontes-Horner D, Marquez-Sandoval F, Martin-del-Campo F, Vizmanos-Lamotte B, Sandoval-Rodriguez A, Armendariz-Borunda J, et al. Effect of a symbiotic gel (Lactobacillus acidophilus + Bifidobacterium lactis + inulin) on presence and severity of gastrointestinal symptoms in hemodialysis patients. Journal of Renal Nutrition 2015;25(3):284-91. [MEDLINE: ] - PubMed
Wang 2015a {published data only}
-
- Wang IK, Wu YY, Yang YF, Ting IW, Lin CC, Yen TH, et al. The effect of probiotics on serum levels of cytokine and endotoxin in peritoneal dialysis patients: a randomised, double-blind, placebo-controlled trial. Beneficial Microbes 2015;6(4):423-30. [MEDLINE: ] - PubMed
References to studies excluded from this review
Afaghi 2016 {published data only}
Cruz‐Mora 2014 {published data only}
-
- Cruz-Mora J, Martinez-Hernandez NE, Martin del Campo-Lopez F, Viramontes-Horner D, Vizmanos-Lamotte B, Munoz-Valle JF, et al. Effects of a symbiotic on gut microbiota in Mexican patients with end-stage renal disease. Journal of Renal Nutrition 2014;24(5):330-5. [MEDLINE: ] - PubMed
Eguchi 2011 {published data only}
-
- Eguchi S, Takatsuki M, Hidaka M, Soyama A, Ichikawa T, Kanematsu T. Perioperative synbiotic treatment to prevent infectious complications in patients after elective living donor liver transplantation: a prospective randomized study. American Journal of Surgery 2011;201(4):498-502. [MEDLINE: ] - PubMed
Ferrara 2009 {published data only}
-
- Ferrara P, Romaniello L, Vitelli O, Gatto A, Serva M, Cataldi L. Cranberry juice for the prevention of recurrent urinary tract infections: a randomized controlled trial in children. Scandinavian Journal of Urology & Nephrology 2009;43(5):369-72. [MEDLINE: ] - PubMed
Firouzi 2015a {published data only}
Fitschen 2015 {published data only}
-
- Fitschen PJ, Kistler B, Wu PT, Chung HR, Jeong JH, Phillips S, et al. Effects of intradialytic whey protein supplementation on body composition in non-malnourished hemodialysis patients [abstract no: SA-PO543]. Journal of the American Society of Nephrology 2012;23(Abstract Suppl):763A.
-
- Tomayko E, Wu PT, Chung HR, Fitschen P, Kistler B, Yudell B, et al. Intradialytic protein supplementation attenuates dialysis-associated inflammation and reduces co-morbid disease risk [abstract no: SA-OR401]. Journal of the American Society of Nephrology 2011;22(Abstract Suppl):96A.
-
- Tomayko E, Yudell B, Jeanes E, Kistler B, Fitschen P, Jeong JH, et al. Intradialytic protein supplementation improves co-morbid disease risk in hemodialysis patients [abstract]. FASEB Journal 2012;26(Suppl 1):na. [EMBASE: 70854879]
-
- Tomayko EJ, Kistler BM, Fitschen PJ, Wilund KR. Intradialytic protein supplementation reduces inflammation and improves physical function in maintenance hemodialysis patients. Journal of Renal Nutrition 2015;25(3):276-83. [MEDLINE: ] - PubMed
Fortes 2020 {published data only}
-
- Fortes PM, Teles Filho RV, Azevedo LH, Queiroz VC, da Costa PS. Inflammatory cytokines and lipid profile in children and adolescents with nephrotic syndrome receiving L. Plantarum: a randomized, controlled feasibility trial. Revista Da Associacao Medica Brasileira 2020;66(11):1487-92. [MEDLINE: ] - PubMed
Grat 2017 {published data only}
-
- Grat M, Krawczyk M, Wronka K, Lewandowski Z, Grat K, Krasnodebski M, et al. Impact of pre-transplant use of probiotics on allograft function after liver transplantation: post-hoc analysis of a randomized controlled trial [abstract]. HPB 2018;20(Suppl 2):S798. [EMBASE: 2001142904]
-
- Grat M, Wronka KM, Lewandowski Z, Grat K, Krasnodebski M, Stypulkowski J, et al. Effects of continuous use of probiotics before liver transplantation: a randomized, double-blind, placebo-controlled trial. Clinical Nutrition 2017;36(6):1530-9. [MEDLINE: ] - PubMed
Hassan 2017 {published data only}
-
- Hassan K, Armaly Z, Hassan D, Khayr M, Rubinchik I, Khazim K, et al. Does whey protein supplementation affect systemic inflammation in hypoalbuminemic peritoneal dialysis patients? [abstract no: SP496]. Nephrology Dialysis Transplantation 2017;32(Suppl 3):iii295-6. [EMBASE: 617291379]
Jiang 2021a {published data only}
-
- Liu S, Jiang H. Probiotic dietary supplementation in hemodialysis patients: a double-blind,randomized, placebo-controlled trial [abstract no: FR-OR128]. Journal of the American Society of Nephrology 2018;29(Abstract Suppl):73. [EMBASE: 633737360]
Lin 2021 {published data only}
-
- Lin W, Jiang C, Yu H, Wang L, Li J, Liu X, et al. The effects of fushen granule on the composition and function of the gut microbiota during peritoneal dialysis-related peritonitis. Phytomedicine 2021;86:153561. [MEDLINE: ] - PubMed
Orr 2016 {published data only}
-
- Orr DW, Myint H, Murphy R. Probiotic supplementation after very low calorie diet does not aid improvement of the metabolic syndrome or maintenance of weight loss post liver transplant. A randomised double-blind placebo controlled trial [abstract no: 214]. Hepatology 2016;64(1 Suppl 1):113-4A. [EMBASE: 612594308]
Pavan 2016 {published data only}
-
- Pavan M. Influence of prebiotic and probiotic supplementation on the progression of chronic kidney disease. Minerva Urologica e Nefrologica 2016;68(2):222-6. [MEDLINE: ] - PubMed
PrePro 2018 {published data only}
-
- Mallick S, Kathirvel M, Thillai M, Sethi P, Durairaj MS, Nair K, et al. PrePro Trial: randomized double-blind placebo controlled trial to analyze the effect of synbiotics on infectious complications following living donor liver transplantion [CTRI no. - CTRI/2017/09/009869]. HPB 2018;20(Suppl 2):S283-4. [EMBASE: 2000962392]
-
- Mallick S, Mathew JS, Binoj S, Unnikrishnan G, Menon RN, Dinesh B, et al. PrePro trial: a randomized double blind placebo controlled trial to analyze the effect of synbiotics on infectious complications following living donor liver transplant (LDLT). [CTRI/2017/09/009869]. Transplantation 2018;102(5 Suppl 1):63. [EMBASE: 622538666]
ProLow CKD 2022 {published data only}
-
- De Mauri A, Carrera D, Bagnati M, Rolla R, Vidali M, Chiarinotti D, et al. Probiotics-supplemented low-protein diet for microbiota modulation in patients with advanced chronic kidney disease (ProLowCKD): results from a placebo-controlled randomized trial. Nutrients 2022;14(8):1637. [MEDLINE: ] - PMC - PubMed
Rayes 2002 {published data only}
-
- Rayes N, Seehofer D, Hansen S, Boucsein K, Muller AR, Serke S, et al. Early enteral supply of lactobacillus and fiber versus selective bowel decontamination: a controlled trial in liver transplant recipients. Transplantation 2002;74(1):123-7. [MEDLINE: ] - PubMed
Rayes 2002a {published data only}
-
- Rayes N, Seehofer D, Muller AR, Hansen S, Bengmark S, Neuhaus P. Influence of probiotics and fibre on the incidence of bacterial infections following major abdominal surgery - results of a prospective trial [Einfluss von Probiotika und Ballaststoffen auf die Inzidenz bakterieller Infektionen nach viszeralchirurgischen Eingriffen - Ergebnisse einer prospektiven Studie]. Zeitschrift fur Gastroenterologie 2002;40(10):869-76. [MEDLINE: ] - PubMed
Rayes 2005 {published data only}
-
- Rayes N, Seehofer D, Theruvath T, Schiller RA, Langrehr JM, Jonas S, et al. Supply of pre- and probiotics reduces bacterial infection rates after liver transplantation--a randomized, double-blind trial. American Journal of Transplantation 2005;5(1):125-30. [MEDLINE: ] - PubMed
Saxena 2022 {published data only}
-
- Saxena A, Jacob CK, Sreenivasa S, Veerappan I, Mahaldar AR, Gupta A, et al. A prospective, double-blind, randomized, placebo-controlled interventional study to evaluate the safety and efficacy of enzobiotics in pre-dialysis CKD patients [abstract no: PO2628]. Journal of the American Society of Nephrology 2020;31(Abstract Suppl):B5. [EMBASE: 633696629]
Tayebi‐Khosroshahi 2016 {published data only}
-
- Tayebi-Khosroshahi H, Habibzadeh A, Niknafs B, Ghotaslou R, Yeganeh Sefidan F, Ghojazadeh M, et al. The effect of lactulose supplementation on fecal microflora of patients with chronic kidney disease; a randomized clinical trial. Journal of Renal Injury Prevention 2016;5(3):162-7. [MEDLINE: ] - PMC - PubMed
Wang 2018e {published data only}
-
- Wang M, Liu H. Dietary advanced glycation end products restriction effects on intestinal bacterial flora and microinflammation state in maintenance hemodialysis patients [abstract no: SA-PO1013]. Journal of the American Society of Nephrology 2019;30(Abstract Suppl):1027. [EMBASE: 633768911]
-
- Wang M. Dietary advanced glycation end products restriction effects on intestinal bacterial flora and microinflammation state in maintenance hemodialysis patients [abstract no: SP664]. Nephrology Dialysis Transplantation 2018;33(Suppl 1):i569-70. [EMBASE: 622605572]
References to studies awaiting assessment
Chen 2019b {published data only}
-
- Chen X, Chen S, Huang L, Mao Z. Probiotics decreased concentrations of indoxylsulphate in PD patients [abstract no: SA-PO954]. Journal of the American Society of Nephrology 2019;30(Abstract Suppl):1011. [EMBASE: 633767865]
Mady 2018 {published data only}
-
- Mady G, Sarhan I, Shawki S, Halim A, Mahanna N, Abdallah M. Effect of probiotics on serum indoxyl sulphate in hemodialysis patients [abstract]. Hemodialysis International 2018;22(1):A15. [EMBASE: 620701234]
Marks 2010 {published data only}
-
- Marks WH, Spinelli TY, Olmstead SF. Probiotics to reduce immunosuppression-associated diarrhea following kidney transplantation - a prospective double-blinded randomized placebo-controlled trial [abstract no: 96]. American Journal of Transplantation 2010;10(Suppl 4):68. [EMBASE: 70463457]
Ogawa 2020 {published data only}
-
- Ogawa T, Hosoda Y, Horimoto A, Nishizawa Y, Omae K, Nagano N. Probiotic bio-three decreases serum phosphate levels with involvement of bacteria in gut microbioma in hemodialysis patients: A prospective, randomized, double-blind, placebo-contolled study [abstract no: SO055]. Nephrology Dialysis Transplantation 2020;35(Suppl 3):iii61. [EMBASE: 633421401]
Prado 2020 {published data only}
-
- Prado L, Aguilar K, Arellano J, Mariscal L, Campos I, Tuz F, et al. Lipid profile improvement in chronic kidney disease patients using a symbiotic supplement with agave inulin, Lactobacillus rhamnosus and Bifidobacterium longum. A post-hoc analysis from a randomized controlled trial [abstract no: 21]. Blood Purification 2020;49(1-2):243. [EMBASE: 631196086]
Ranganathan 2019a {published data only}
-
- Ranganathan N, Vyas UN, Ranganathan P, Irvin A, Weinberg AD. A double-blind, randomized, placebo-controlled with an open-label rollover extension phase 2/3 clinical trial to evaluate safety and efficacy of US-APR2020 in subjects with CKD stage IV [abstract no: PUB036]. Journal of the American Society of Nephrology 2020;31(Abstract Suppl):801. [EMBASE: 633696783]
-
- Ranganathan N, Vyas UN, Ranganathan P, Irvin A, Weinberg AD. Kibow multisite hope study dialysis randomized clinical trial protocol: a unique double-blind placebo-controlled cross-over design using renadyl standard-care therapy (n=100, 5 sites in the United States) [abstract no: PUB134]. Journal of the American Society of Nephrology 2019;30(Abstract Suppl):1108. [EMBASE: 633771192]
-
- Ranganathan N, Vyas UN, Ranganathan P, Irvin A, Weinberg AD. Kibow multisite hope study-CKD IV randomized clinical trial protocol: a unique double-blind placebo-controlled cross-over design using renadyl with standard-of-care therapy (n=500-600, 20-25 sites in the United States) [abstract no: FR-PO339]. Journal of the American Society of Nephrology 2019;30(Abstract Suppl):524-5. [EMBASE: 633768784]
Soliman 2018 {published data only}
-
- Soliman Ahmed Y, Ibrahim Sarhaan E, Shaker Mehanna N, Saeed Hassan M, Abd-El Nasier Abd-El Gawad M, Nagdy Madbouli N. The effect of synbiotics on serum indoxyl sulfate in maintenance haemodialysis patients [abstract]. QJM 2018;111(Suppl 1):i84-5. [EMBASE: 631035999]
-
- Soliman Y, Sarhan I, Mahanna N, Saeed M, Abd-ElNasier M, Nagdy N. The effect of synbiotic on serum indoxyl sulfate in maintenance hemodialysis patients [abstract]. Hemodialysis International 2018;22(1):A15. [EMBASE: 620701232]
Takayama 2003 {published data only}
-
- Takayama F, Enomoto A, Aoyama I. Oral administration of bifidobacteria in gastro-resistant seamless capsule reduces serum levels of indoxyl sulfate in patients on hemodialysis [abstract no: T297]. Nephrology Dialysis Transplantation 2002;17(Suppl 1):271. [CENTRAL: CN-00644305]
-
- Takayama F, Taki K, Niwa T. Bifidobacterium in gastro-resistant seamless capsule reduces serum levels of indoxyl sulfate in patients on hemodialysis. American Journal of Kidney Diseases 2003;41(3 Suppl 1):S142-5. [MEDLINE: ] - PubMed
TCTR20220317007 {published data only}
-
- Amelioration of uremic toxin indoxyl sulfate by oral chito-oligosaccharide in predialysis patients: a randomized controlled trial. https://trialsearch.who.int/Trial2.aspx?TrialID=TCTR20220317007 (date posted 1 March 2022). - PubMed
-
- Sirisuksakun C, Thimachai P, Tasanavipas P, Siriwattanasit N, Inkong P, Varothai N, et al. Amelioration of uremic toxin indoxyl sulfate by oral chito-oligosaccharide in predialysis patients: a randomized controlled trial [abstract no: SA-PO901]. Journal of the American Society of Nephrology 2022;33:852.
Wu 2012 {published data only}
-
- Wu AB, Wang MC, Chang YT, Tseng CC. Probiotics decrease the incidence of gram negative peritonitis in patients on peritoneal dialysis [abstract no: SA-PO864]. Journal of the American Society of Nephrology 2012;23(Abstract Suppl):840A.
Younes 2006 {published data only}
-
- Younes H, Egret N, Hadj-Abdelkader M, Remesy C, Demigne C, Gueret C, et al. Fermentable carbohydrate supplementation alters nitrogen excretion in chronic renal failure. Journal of Renal Nutrition 2006;16(1):67-74. [MEDLINE: ] - PubMed
Zhang 2019a {published data only}
-
- Zhang Q. Effect of probiotic on gut microbiota in peritoneal dialysis patient with protein-energy wasting: 1 year follow up of a randomised controlled trial [abstract no: P0917]. European Journal of Immunology 2019;49(Suppl 3):1127-8. [EMBASE: 631545578]
References to ongoing studies
ChiCTR2200061930 {published data only}
-
- Effects of non-protein energy supplements and exercise on protein-energy wasting and gut microbes in maintenance hemodialysis patients. https://trialsearch.who.int/Trial2.aspx?TrialID=ChiCTR2200061930 (date posted 11 July 2022).
ChiCTR2200064821 {published data only}
-
- Clinical effect and mechanism of modified Shenling Baizhu powder on peritoneal dialysis. https://trialsearch.who.int/Trial2.aspx?TrialID=ChiCTR2200064821 (date posted 19 October 2022).
ChiCTR2300070381 {published data only}
-
- Effects of dietary fiber-added diets on protein-binding toxoids and microinflammatory states in patients with chronic kidney disease. https://trialsearch.who.int/Trial2.aspx?TrialID=ChiCTR2300070381 (date posted 11 April 2023).
ChiCTR2300072339 {published data only}
-
- Probiotics improve immune metabolism in maintenance hemodialysis patients by regulating intestinal flora. https://trialsearch.who.int/Trial2.aspx?TrialID=ChiCTR2300072339 (date posted 9 June 2023).
CTRI/2022/02/040296 {published data only}
-
- A study to assess the effect of probiotic in prevention of progression of kidney disease in patients with chronic kidney disease [A controlled randomized study to evaluate the effect of probiotic on uremic solutes,inflammatory status and oxidative stress in chronic kidney disease subjects - PRO-URE-CKD]. https://trialsearch.who.int/Trial2.aspx?TrialID=CTRI/2022/02/040296 (date posted 15 February 2022).
CTRI/2022/11/047767 {published data only}
-
- Global phase 2 clinical trial to evaluate safety and efficacy of US-APR2020 in subjects with chronic kidney disease stage IV. https://trialsearch.who.int/Trial2.aspx?TrialID=CTRI/2022/11/047767 (date posted 29 November 2022).
Ghoreishy 2022 {published data only}
-
- Ghoreishy SM, Shirzad N, Nakhjavani M, Esteghamati A, Djafarian K, Esmaillzadeh A. Effect of daily consumption of probiotic yoghurt on albumin to creatinine ratio, eGFR and metabolic parameters in patients with type 2 diabetes with microalbuminuria: study protocol for a randomised controlled clinical trial. BMJ Open 2022;12(3):e056110. [MEDLINE: ] - PMC - PubMed
Headley 2020 {published data only}
-
- Headley SA, Chapman DJ, Germain MJ, Evans EE, Hutchinson J, Madsen KL, et al. The effects of 16-weeks of prebiotic supplementation and aerobic exercise training on inflammatory markers, oxidative stress, uremic toxins, and the microbiota in pre-dialysis kidney patients: a randomized controlled trial-protocol paper. BMC Nephrology 2020;21(1):517. [MEDLINE: ] - PMC - PubMed
IRCT20100223003408N5 {published data only}
-
- Ekramzadeh M, Azad F, Hamidianshirazi M, Mazloomi SM, Shafiee M. Efficacy of fortified synbiotic dessert on malnutrition and oxidative stress outcomes in hemodialysis patients: a randomized double-blind controlled trial [abstract]. American Journal of Kidney Diseases 2023;81(4):S113. [DOI: 10.1053/j.ajkd.2023.01.390] - DOI
-
- Ekramzadeh M. Synbiotic dessert fortified with vitamin D and dalcium in hemodialysis patients [Effect of synbiotic dessert fortified with vitamin d and calcium on nutritional indices, inflammation, oxidative stress, malnutrition and quality of life in hemodialysis patients]. en.irct.ir/trial/35600 (date posted 6 April 2019).
IRCT20131013014994N7 {published data only}
-
- The effect of synbiotic supplementation on plasma levels of Advanced Glycation End Products and cardiovascular risk factors in hemodialysis patients: a double-blind clinical trial. https://trialsearch.who.int/Trial2.aspx?TrialID=IRCT20131013014994N7 (date posted 27 April 2022). - PMC - PubMed
IRCT20160206026390N11 {published data only}
-
- Ghaedi E. Probiotics and children under hemodialysis treatment [Evaluation of the efficacy of probiotic supplementation on bio-markers of inflammation and oxidative stress in pediatric patients under treatment with hemodialysis: randomized double blind clinical trial]. en.irct.ir/trial/35899 (date posted 10 June 2019).
IRCT20230608058421N1 {published data only}
-
- The effect of probiotics on patients with end-stage renal failure. https://trialsearch.who.int/Trial2.aspx?TrialID=IRCT20230608058421N1 (date posted 29 June 2023).
KCT0007834 {published data only}
-
- The effect of carboneceous oral adsorbent and synbiotics on the gut microbiome and muscle health in chronic kidney disease patients. https://trialsearch.who.int/Trial2.aspx?TrialID=KCT0007834 (date posted 21 October 2022).
Mitrovic 2023 {published data only}
-
- Mitrovic M, Stankovic-Popovic V, Tolinacki M, Golic N, Sokovic Bajic S, Veljovic K, et al. The impact of synbiotic treatment on the levels of gut-derived uremic toxins, inflammation, and gut microbiome of chronic kidney disease patients- a randomized trial. Journal of Renal Nutrition 2023;33(2):278-88. [MEDLINE: ] - PubMed
NCT03770611 {published data only}
-
- Cueto-Manzano A. Effect of prebiotics and/or probiotics on uremic toxins and inflammation markers in peritoneal dialysis patients [Effect of a nutritional supplement of probiotics and/or prebiotics vs placebo on serum concentrations of uremic toxins and inflammatory cytokines in automated peritoneal dialysis patients]. www.clinicaltrials.gov/show/NCT03770611 (date posted 10 December 2018).
NCT03789708 {published data only}
-
- Elamin S. Effects of gum arabic supplementation in hemodialysis patients [Study of some pharmacological and biochemical effects of gum arabic supplementation to hemodilaysis patients]. www.clinicaltrials.gov/show/NCT03789708 (date posted 31 December 2018).
NCT03924089 {published data only}
-
- Olveira G. Oral nutritional supplement on nutritional and functional status, and biomarkers in malnourished hemodialysis patients (RENACARE) [Effect of an oral nutritional supplement on nutritional and functional status, biological markers (inflammation and oxidative stress, intestinal microbiota, circulating microRNA And its target genes) in malnourished hemodialysis patients]. clinicaltrials.gov/show/NCT03924089 (date posted 23 April 2019).
NCT05183737 {published data only}
-
- Effects of microencapsulated propolis and turmeric in patients with chronic kidney disease. https://clinicaltrials.gov/show/NCT05183737 (date posted 11 January 2022).
NCT05336305 {published data only}
-
- Polydextrose for patients with chronic kidney disease. https://clinicaltrials.gov/show/NCT05336305 (date posted 20 April 2022).
NCT05359094 {published data only}
-
- Probiotic supplements in chronic kidney disease. https://clinicaltrials.gov/show/NCT05359094 (date posted 3 May 2022).
NCT05540431 {published data only}
-
- Evaluation of protective effect of activated charcoal and probiotic against progression of chronic kidney disease. https://clinicaltrials.gov/show/NCT05540431 (date posted 14 September 2022).
NCT05674981 {published data only}
-
- To evaluate the beneficial effect of probiotics on DKD patients and the role of gut microbiota modulation. https://clinicaltrials.gov/show/NCT05674981 (date posted 9 January 2023).
NCT05724511 {published data only}
-
- The effect of probiotics on depression syndrome and risk factors of cardiovascular disease in hemodialysis patients. https://clinicaltrials.gov/show/NCT05724511 (date posted 13 February 2023).
NCT05835648 {published data only}
-
- Effect of dietary fiber on non-dialysis patients with chronic kidney disease. https://clinicaltrials.gov/show/NCT05835648 (date posted 28 April 2023).
RBR‐3WRNF {published data only}
-
- Moraes da Silva A. Effect of prebiotics on intestinal transit and inflammation of patients with pre-dialytic chronic renal disease. www.ensaiosclinicos.gov.br/rg/RBR-3wrnrf/ (date posted 13 December 2018).
Additional references
Aron‐Wisnewsky 2016
-
- Aron-Wisnewsky J, Clement K. The gut microbiome, diet, and links to cardiometabolic and chronic disorders. Nature Reviews Nephrology 2016;12(3):169-81. [MEDLINE: ] - PubMed
Azad 2018
Balshem 2011
-
- Balshem H, Helfand M, Schünemann HJ, Oxman AD, Kunz R, Brozek J, et al. GRADE guidelines: 3. Rating the quality of evidence. Journal of Clinical Epidemiology 2011;64(4):401-6. [MEDLINE: ] - PubMed
Beerepoot 2016
Bromberg 2015
-
- Bromberg JS, Fricke WF, Brinkman CC, Simon T, Mongodin EF. Microbiota - implications for immunity and transplantation. Nature Reviews Nephrology 2015;11(6):342-53. [MEDLINE: ] - PubMed
Cao 2022
Chan 2020
Chen 2022
-
- Chen C, Wang J, Li J, Zhang W, Ou S. Probiotics, prebiotics, and synbiotics for patients on dialysis: a systematic review and meta-analysis of randomized controlled trials. Journal of Renal Nutrition 2023;33(1):129-39. [DOI: ] - PubMed
Cremon 2018
-
- Cremon C, Barbaro MR, Ventura M, Barbara G. Pre- and probiotic overview. Current Opinion in Pharmacology 2018;43:87-92. [MEDLINE: ] - PubMed
Davani‐Davari 2019
FAO/WHO 2002
-
- Joint FAO/WHO Working Group Report on Drafting Guidelines for the Evaluation of Probiotics in Food London, Ontario, Canada, April 30 and May 1, 2002. Guidelines for the evaluation of probiotics in food. www.who.int/foodsafety/fs_management/en/probiotic_guidelines.pdf (last accessed 7 May 2020).
GBD 2017
-
- GBD 2017 Causes of Death Collaborators. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980-2017: a systematic analysis for the Global Burden of Disease Study 2017 [Erratum in: Lancet. 2019 Jun 22;393(10190):e44; PMID: 31232379] [Erratum in: Lancet. 2018 Nov 17;392(10160):2170; PMID: 31329658]. Lancet 2018;392(10159):1736-88. [MEDLINE: ] - PMC - PubMed
Gibson 2017
-
- Gibson GR, Hutkins R, Sanders ME, Prescott SL, Reimer RA, Salminen SJ, et al. Expert consensus document: the International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nature Reviews. Gastroenterology & Hepatology 2017;14(8):491-502. [MEDLINE: ] - PubMed
GRADE 2008
GRADE 2011
-
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011;64(4):383-94. [MEDLINE: ] - PubMed
Higgins 2003
Higgins 2022
-
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al. Cochrane Handbook for Systematic Reviews of Interventions version 6.3 (updated February 2022). Cochrane, 2022. Available from www.training.cochrane.org/handbook.
Hill 2016
Jha 2013
-
- Jha V, Garcia-Garcia G, Iseki K, Li Z, Naicker S, Plattner B, et al. Chronic kidney disease: global dimension and perspectives [Erratum in: Lancet. 2013 Jul 20;382(9888):208]. Lancet 2013;382(9888):260-72. [MEDLINE: ] - PubMed
Kato 2008
KDIGO 2013
-
- Stevens PE, Levin A. Evaluation and management of chronic kidney disease: synopsis of the kidney disease: improving global outcomes 2012 clinical practice guideline. Annals of Internal Medicine 2013;158(11):825-30. [MEDLINE: ] - PubMed
Lehto 2018
Lewis 1997
-
- Lewis SJ, Heaton KW. Stool form scale as a useful guide to intestinal transit time. Scandinavian Journal of Gastroenterology 1997;32(9):920-4. [MEDLINE: ] - PubMed
Luyckx 2018
Mafra 2019
March 2020
-
- March DS, Jones AW, Bishop NC, Burton JO. The efficacy of prebiotic, probiotic, and synbiotic supplementation in modulating gut-derived circulatory particles associated with cardiovascular disease in individuals receiving dialysis: a systematic review and meta-analysis of randomized controlled trials. Journal of Renal Nutrition 2020;30(4):347-59. [PMID: ] - PubMed
McFarlane 2019
-
- McFarlane C, Ramos CI, Johnson DW, Campbell KL. Prebiotic, probiotic, and synbiotic supplementation in chronic kidney disease: a systematic review and meta-analysis. Journal of Renal Nutrition 2019;9(3):209-20. [PMID: ] - PubMed
Pan 2018
-
- Pan W, Kang Y. Gut microbiota and chronic kidney disease: implications for novel mechanistic insights and therapeutic strategies. International Urology & Nephrology 2018;50(2):289-99. [MEDLINE: ] - PubMed
Sampai‐Maia 2016
-
- Sampaio-Maia B, Simões-Silva L, Pestana M, Araujo R, Soares-Silva IJ. The role of the gut microbiome on chronic kidney disease. Advances in Applied Microbiology 2016;96:65-94. [PMID: ] - PubMed
Schünemann 2022a
-
- Schünemann HJ, Higgins JP, Vist GE, Glasziou P, Akl EA, Skoetz N, et al. Chapter 14: Completing ‘Summary of findings’ tables and grading the certainty of the evidence. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.3 (updated February 2022). Cochrane, 2022. www.training.cochrane.org/handbook.
Schünemann 2022b
-
- Schünemann HJ, Vist GE, Higgins JP, Santesso N, Deeks JJ, Glasziou P, et al. Chapter 15: Interpreting results and drawing conclusions. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.3 (updated February 2022). Cochrane, 2022. Available from www.training.cochrane.org/handbook.
SONG 2017
-
- SONG Initiative. The SONG Handbook Version 1.0. www.songinitiative.org/reports-and-publications/ 2017.
Valdes 2018
Zheng 2020
-
- Zheng JH, Guo J, Wang Q, Wang L, Wang Y, Zhang F, et al. Probiotics, prebiotics, and synbiotics for the improvement of metabolic profiles in patients with chronic kidney disease: A systematic review and meta-analysis of randomized controlled trials. Critical Reviews in Food Science & Nutrition 2020;61(4):577–98. [PMID: ] - PubMed
References to other published versions of this review
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

