Promoting Homogeneous Zinc-Ion Transfer Through Preferential Ion Coordination Effect in Gel Electrolyte for Stable Zinc Metal Batteries
- PMID: 37870210
- PMCID: PMC10700204
- DOI: 10.1002/advs.202304915
Promoting Homogeneous Zinc-Ion Transfer Through Preferential Ion Coordination Effect in Gel Electrolyte for Stable Zinc Metal Batteries
Abstract
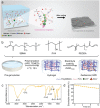
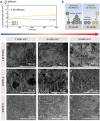
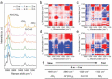
Aqueous zinc metal batteries (AZMBs) are emerging energy storage systems that are poised to replace conventional lithium-ion batteries owing to their intrinsic safety, facile manufacturing process, economic benefits, and superior ionic conductivity. However, the issues of inferior anode reversibility and dendritic plating during operation remain challenging for the practical use of AZMBs. Herein, a gel electrolyte based on zwitterionic poly(sulfobetaine methacrylate) (poly(SBMA)) dissolved with different concentrations of ZnSO4 is proposed. Two-dimensional correlation spectroscopy based on Raman analysis reveals an enhanced interaction priority between the polar groups in SBMA and the dissolved ions as electrolyte concentration increases, which establishes a robust interaction and renders homogeneous ion distribution. Attributable to the modified coordination, zwitterionic gel polymer electrolyte with 5 mol kg-1 of ZnSO4 (ZGPE-5) facilitates stable zinc deposition and improves anode reversibility. By taking advantage of preferential coordination, a symmetrical cell evaluation employing ZGPE-5 demonstrates a cycle life over 3600 h, where ZGPE-5 also exerts a beneficial effect on the full cell cycling when assembled with Zn0.25 V2 O5 cathode. This study elucidates changes in the internal ion behavior that are dependent on electrolyte concentrations and pave the way for durable AZMBs.
Keywords: aqueous rechargeable batteries; ion coordination; preferential coordination; zinc metal anodes; zwitterionic gel electrolytes.
© 2023 The Authors. Advanced Science published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Popovich N. D., Rajagopal D., Tasar E., Phadke A., Nat. Energy 2021, 6, 1017.
-
- Chen Z. X., Zhao M., Hou L. P., Zhang X. Q., Li B. Q., Huang J. Q., Adv. Mater 2022, 34, 2201555.
-
- Mrozik W., Rajaeifar M. A., Heidrich O., Christensen P., Energy Environ. Sci. 2021, 14, 6099.
-
- Yang Y., Okonkwo E. G., Huang G., Xu S., Sun W., He Y., Energy Storage Mater. 2021, 36, 186.
Grants and funding
LinkOut - more resources
Full Text Sources
