Single-Cell RNA-Sequencing Provides Insight into Skeletal Muscle Evolution during the Selection of Muscle Characteristics
- PMID: 37870215
- PMCID: PMC10724408
- DOI: 10.1002/advs.202305080
Single-Cell RNA-Sequencing Provides Insight into Skeletal Muscle Evolution during the Selection of Muscle Characteristics
Abstract
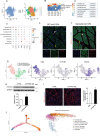
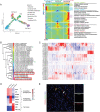
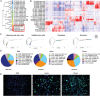
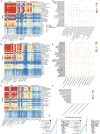
Skeletal muscle comprises a large, heterogeneous assortment of cell populations that interact to maintain muscle homeostasis, but little is known about the mechanism that controls myogenic development in response to artificial selection. Different pig (Sus scrofa) breeds exhibit distinct muscle phenotypes resulting from domestication and selective breeding. Using unbiased single-cell transcriptomic sequencing analysis (scRNA-seq), the impact of artificial selection on cell profiles is investigated in neonatal skeletal muscle of pigs. This work provides panoramic muscle-resident cell profiles and identifies novel and breed-specific cells, mapping them on pseudotime trajectories. Artificial selection has elicited significant changes in muscle-resident cell profiles, while conserving signs of generational environmental challenges. These results suggest that fibro-adipogenic progenitors serve as a cellular interaction hub and that specific transcription factors identified here may serve as candidate target regulons for the pursuit of a specific muscle phenotype. Furthermore, a cross-species comparison of humans, mice, and pigs illustrates the conservation and divergence of mammalian muscle ontology. The findings of this study reveal shifts in cellular heterogeneity, novel cell subpopulations, and their interactions that may greatly facilitate the understanding of the mechanism underlying divergent muscle phenotypes arising from artificial selection.
Keywords: fibro-adipogenic progenitors; muscle characteristics; neonatal myogenesis; pigs, single-cell RNA-sequencing.
© 2023 The Authors. Advanced Science published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Ai H., Fang X., Yang B., Huang Z., Chen H., Mao L., Zhang F., Zhang L., Cui L., He W., Yang J., Yao X., Zhou L., Han L., Li J., Sun S., Xie X., Lai B., Su Y., Lu Y., Yang H., Huang T., Deng W., Nielsen R., Ren J., Huang L., Nat. Genet. 2015, 47, 217. - PubMed
-
- a) Zecchini S., Giovarelli M., Perrotta C., Morisi F., Touvier T., Di Renzo I., Moscheni C., Bassi M. T., Cervia D., Sandri M., Clementi E., De Palma C., Autophagy 2019, 15, 58; - PMC - PubMed
- b) Murphy M., Kardon G., Curr. Top. Dev. Biol. 2011, 96, 21621065; - PMC - PubMed
- c) Stockdale F. E., Dev Biol. 1992, 154, 284. - PubMed
-
- Huang Y., Zhou L., Zhang J., Liu X., Zhang Y., Cai L., Zhang W., Cui L., Yang J., Ji J., Xiao S., Ai H., Chen C., Ma J., Yang B., Huang L., Meat Sci. 2020, 168, 108182. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
