Association of hepatitis delta virus with liver morbidity and mortality: A systematic literature review and meta-analysis
- PMID: 37870278
- PMCID: PMC11019996
- DOI: 10.1097/HEP.0000000000000642
Association of hepatitis delta virus with liver morbidity and mortality: A systematic literature review and meta-analysis
Abstract
Background and aims: Studies have suggested that patients with chronic hepatitis B, either co- or superinfected, have more aggressive liver disease progression than those with the HDV. This systematic literature review and meta-analysis examined whether HDV RNA status is associated with increased risk of advanced liver disease events in patients who are HBsAg and HDV antibody positive.
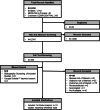
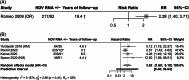
Approach and results: A total of 12 publications were included. Relative rates of progression to advanced liver disease event for HDV RNA+/detectable versus HDV RNA-/undetectable were extracted for analysis. Reported OR and HRs with 95% CI were pooled using the Hartung-Knapp-Sidik-Jonkman method for random-effects models. The presence of HDV RNA+ was associated with an increased risk of any advanced liver disease event [random effect (95% CI): risk ratio: 1.48 (0.93, 2.33); HR: 2.62 (1.55, 4.44)]. When compared to the patients with HDV RNA- status, HDV RNA+ was associated with a significantly higher risk of progressing to compensated cirrhosis [risk ratio: 1.74 (1.24, 2.45)] decompensated cirrhosis [HR: 3.82 (1.60, 9.10)], HCC [HR: 2.97 (1.87, 4.70)], liver transplantation [HR: 7.07 (1.61, 30.99)], and liver-related mortality [HR: 3.78 (2.18, 6.56)].
Conclusions: The patients with HDV RNA+ status have a significantly greater risk of liver disease progression than the patients who are HDV RNA-. These findings highlight the need for improved HDV screening and linkage to treatment to reduce the risk of liver-related morbidity and mortality.
Copyright © 2023 The Author(s). Published by Wolters Kluwer Health, Inc.
Conflict of interest statement
Robert G. Gish consults, advises, and is on the speakers’ bureau for AbbVie, Genentech, Gilead, and Intercept. He consults, advises, and owns stock in Genlantis, HepQuant, and HepaTX. He consults and advises Arrowhead, Dynavax, Enyo, Helios, Janssen, Merck, and Pfizer. He consults and is on the speakers’ bureau for Eisai. He consults and has other interests in Topography Health. He consults for Abbott, Altimmune, Antios, Eiger, Fibronostics, Fujifilm/Wako, Gerson Lehrman Group, Perspectum, and Venatorx. He advises CymaBay, Durect, Prodigy, Quest, and Sonic Incytes. He is on the speakers’ bureau for AstraZeneca and Bristol Myers Squibb. He owns stock in AngioCrine, CoCrystal, Eiger, and RiboSciences. Robert J. Wong consults and received grants from Gilead. He received grants from Exact Sciences and Theratechnologies. Gian Luca Di Tanna consults for Gilead. Ankita Kaushik is employed by and owns stock in Gilead. Chong Kim is employed by Gilead. Nathaniel J. Smith consults for Gilead. He is employed by Maple Health Group. Patrick T.F. Kennedy consults, is on the speakers’ bureau, and received grants from Gilead. He consults for Assembly Bio and GlaxoSmithKline.
Figures




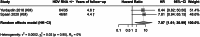

Comment in
-
HDV screening in chronic HBV: An unmet need of growing importance.Hepatology. 2024 May 1;79(5):979-982. doi: 10.1097/HEP.0000000000000722. Epub 2023 Dec 13. Hepatology. 2024. PMID: 38088906 No abstract available.
-
HDV RNA and liver disease progression: What do we know?Hepatology. 2024 May 1;79(5):983-985. doi: 10.1097/HEP.0000000000000663. Epub 2023 Dec 29. Hepatology. 2024. PMID: 38156968 No abstract available.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

