The Glycine N-Methyltransferase Case Study: Another Challenge for QM-Cluster Models?
- PMID: 37870315
- PMCID: PMC11018112
- DOI: 10.1021/acs.jpcb.3c04138
The Glycine N-Methyltransferase Case Study: Another Challenge for QM-Cluster Models?
Abstract
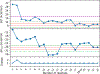
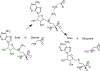
The methyl transfer reaction between SAM and glycine catalyzed by glycine N-methyltransferase (GNMT) was examined using QM-cluster models generated by Residue Interaction Network ResidUe Selector (RINRUS). RINRUS is a Python-based tool that can build QM-cluster models with rules-based processing of the active site residue interaction network. This way of enzyme model-building allows quantitative analysis of residue and fragment contributions to kinetic and thermodynamic properties of the enzyme. Many residue fragments are important for the GNMT catalytic reaction, such as Gly137, Asn138, and Arg175, which interact with the glycine substrate, and Trp30, Asp85, and Tyr242, which interact with the SAM cofactor. Our study shows that active site fragments that interact with the glycine substrate and the SAM cofactor must both be included in the QM-cluster models. Even though the proposed mechanism is a simple one-step reaction, GNMT may be a rather challenging case study for QM-cluster models because convergence in energetics requires models with >350 atoms. "Maximal" QM-cluster models built with either qualitative contact count ranking or quantitative interaction energies from functional group symmetry adapted perturbation theory provide acceptable results. Hence, important residue fragments that contribute to the energetics of the methyl-transfer reaction in GNMT are correctly identified in the RIN. Observations from this work suggest new directions to better establish an effective approach for constructing atomic-level enzyme models.
Figures






References
-
- Sirirak J; Lawan N; Van der Kamp MW; Harvey JN; Mulholland AJ Benchmarking Quantum Mechanical Methods for Calculating Reaction Energies of Reactions Catalyzed by Enzymes. PeerJ Phys. Chem. 2020, 2, e8. 10.7717/peerj-pchem.8. - DOI
-
- Cheng Q; DeYonker NJ; Summers TJ; Agbaglo DA; Suhagia T; Manuel PA GitHub - Natedey/RINRUS: Residue Interaction Network ResidUe Selector (RINRUS) Public Release. Https://Github.Com/Natedey/RINRUS (Accessed 2022-09-14). 2022.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

