Safety and Efficacy of Topiramate in Individuals With Cryptogenic Sensory Peripheral Neuropathy With Metabolic Syndrome: The TopCSPN Randomized Clinical Trial
- PMID: 37870862
- PMCID: PMC10594179
- DOI: 10.1001/jamaneurol.2023.3711
Safety and Efficacy of Topiramate in Individuals With Cryptogenic Sensory Peripheral Neuropathy With Metabolic Syndrome: The TopCSPN Randomized Clinical Trial
Abstract
Importance: Cryptogenic sensory peripheral neuropathy (CSPN) is highly prevalent and often disabling due to neuropathic pain. Metabolic syndrome and its components increase neuropathy risk. Diet and exercise have shown promise but are limited by poor adherence.
Objective: To determine whether topiramate can slow decline in intraepidermal nerve fiber density (IENFD) and/or neuropathy-specific quality of life measured using the Norfolk Quality of Life-Diabetic Neuropathy (NQOL-DN) scale.
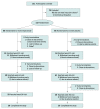
Design, setting, and participants: Topiramate as a Disease-Modifying Therapy for CSPN (TopCSPN) was a double-blind, placebo-controlled, randomized clinical trial conducted between February 2018 and October 2021. TopCSPN was performed at 20 sites in the National Institutes of Health-funded Network for Excellence in Neurosciences Clinical Trials (NeuroNEXT). Individuals with CSPN and metabolic syndrome aged 18 to 80 years were screened and randomly assigned by body mass index (<30 vs ≥30), which is calculated as weight in kilograms divided by height in meters squared. Patients were excluded if they had poorly controlled diabetes, prior topiramate treatment, recurrent nephrolithiasis, type 1 diabetes, use of insulin within 3 months before screening, history of foot ulceration, planned bariatric surgery, history of alcohol or drug overuse in the 2 years before screening, family history of a hereditary neuropathy, or an alternative neuropathy cause.
Interventions: Participants received topiramate or matched placebo titrated to a maximum-tolerated dose of 100 mg per day.
Main outcomes and measures: IENFD and NQOL-DN score were co-primary outcome measures. A positive study was defined as efficacy in both or efficacy in one and noninferiority in the other.
Results: A total of 211 individuals were screened, and 132 were randomly assigned to treatment groups: 66 in the topiramate group and 66 in the placebo group. Age and sex were similar between groups (topiramate: mean [SD] age, 61 (10) years; 38 male [58%]; placebo: mean [SD] age, 62 (11) years; 44 male [67%]). The difference in change in IENFD and NQOL-DN score was noninferior but not superior in the intention-to-treat (ITT) analysis (IENFD, 0.21 fibers/mm per year; 95% CI, -0.43 to ∞ fibers/mm per year and NQOL-DN score, -1.52 points per year; 95% CI, -∞ to 1.19 points per year). A per-protocol analysis excluding noncompliant participants based on serum topiramate levels and those with major protocol deviations demonstrated superiority in NQOL-DN score (-3.69 points per year; 95% CI, -∞ to -0.73 points per year). Patients treated with topiramate had a mean (SD) annual change in IENFD of 0.56 fibers/mm per year relative to placebo (95% CI, -0.21 to ∞ fibers/mm per year). Although IENFD was stable in the topiramate group compared with a decline consistent with expected natural history, this difference did not demonstrate superiority.
Conclusion and relevance: Topiramate did not slow IENFD decline or affect NQOL-DN score in the primary ITT analysis. Some participants were intolerant of topiramate. NQOL-DN score was superior among those compliant based on serum levels and without major protocol deviations.
Trial registration: ClinicalTrials.gov Identifier: NCT02878798.
Conflict of interest statement
Figures

References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- U24 NS107199/NS/NINDS NIH HHS/United States
- P30 DK092926/DK/NIDDK NIH HHS/United States
- U01 NS077179/NS/NINDS NIH HHS/United States
- U10 NS077356/NS/NINDS NIH HHS/United States
- U24 NS107210/NS/NINDS NIH HHS/United States
- U24 NS107198/NS/NINDS NIH HHS/United States
- U24 NS107176/NS/NINDS NIH HHS/United States
- U24 NS107154/NS/NINDS NIH HHS/United States
- U01 NS077352/NS/NINDS NIH HHS/United States
- U24 NS107376/NS/NINDS NIH HHS/United States
- U24 NS107165/NS/NINDS NIH HHS/United States
- U24 NS107181/NS/NINDS NIH HHS/United States
- U01 NS095388/NS/NINDS NIH HHS/United States
- U24 NS107168/NS/NINDS NIH HHS/United States
- U24 NS107128/NS/NINDS NIH HHS/United States
- U24 NS107213/NS/NINDS NIH HHS/United States
- U24 NS107158/NS/NINDS NIH HHS/United States
- U24 NS107205/NS/NINDS NIH HHS/United States
- U24 NS107155/NS/NINDS NIH HHS/United States
- U24 NS107204/NS/NINDS NIH HHS/United States
- U24 NS107197/NS/NINDS NIH HHS/United States
- U24 NS107156/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical

