Three-Year Overall Survival with Tebentafusp in Metastatic Uveal Melanoma
- PMID: 37870955
- PMCID: PMC11188986
- DOI: 10.1056/NEJMoa2304753
Three-Year Overall Survival with Tebentafusp in Metastatic Uveal Melanoma
Abstract
Background: Tebentafusp, a T-cell receptor-bispecific molecule that targets glycoprotein 100 and CD3, is approved for adult patients who are positive for HLA-A*02:01 and have unresectable or metastatic uveal melanoma. The primary analysis in the present phase 3 trial supported a long-term survival benefit associated with the drug.
Methods: We report the 3-year efficacy and safety results from our open-label, phase 3 trial in which HLA-A*02:01-positive patients with previously untreated metastatic uveal melanoma were randomly assigned in a 2:1 ratio to receive tebentafusp (tebentafusp group) or the investigator's choice of therapy with pembrolizumab, ipilimumab, or dacarbazine (control group), with randomization stratified according to the lactate dehydrogenase level. The primary end point was overall survival.
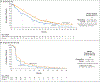
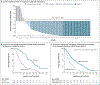
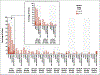
Results: At a minimum follow-up of 36 months, median overall survival was 21.6 months in the tebentafusp group and 16.9 months in the control group (hazard ratio for death, 0.68; 95% confidence interval, 0.54 to 0.87). The estimated percentage of patients surviving at 3 years was 27% in the tebentafusp group and 18% in the control group. The most common treatment-related adverse events of any grade in the tebentafusp group were rash (83%), pyrexia (76%), pruritus (70%), and hypotension (38%). Most tebentafusp-related adverse events occurred early during treatment, and no new adverse events were observed with long-term administration. The percentage of patients who discontinued treatment because of adverse events continued to be low in both treatment groups (2% in the tebentafusp group and 5% in the control group). No treatment-related deaths occurred.
Conclusions: This 3-year analysis supported a continued long-term benefit of tebentafusp for overall survival among adult HLA-A*02:01-positive patients with previously untreated metastatic uveal melanoma. (Funded by Immunocore; IMCgp100-202 ClinicalTrials.gov number, NCT03070392; EudraCT number, 2015-003153-18.).
Copyright © 2023 Massachusetts Medical Society.
Figures
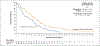
References
-
- Jager MJ, Shields CL, Cebulla CM, et al. Uveal melanoma. Nat Rev Dis Primers 2020; 6: 24. - PubMed
-
- Khoja L, Atenafu EG, Suciu S, et al. Meta-analysis in metastatic uveal melanoma to determine progression free and overall survival benchmarks: an international rare cancers initiative (IRCI) ocular melanoma study. Ann Oncol 2019;30: 1370–80. - PubMed
-
- Carvajal RD, Sacco JJ, Jager MJ, et al. Advances in the clinical management of uveal melanoma. Nat Rev Clin Oncol 2023; 20:9 9–115. - PubMed
-
- Augsburger JJ, Corrêa ZM, Shaikh AH. Effectiveness of treatments for metastatic uveal melanoma. Am J Ophthalmol 2009; 148: 119–27. - PubMed
