Activation of acetyl-CoA synthetase 2 mediates kidney injury in diabetic nephropathy
- PMID: 37870960
- PMCID: PMC10619493
- DOI: 10.1172/jci.insight.165817
Activation of acetyl-CoA synthetase 2 mediates kidney injury in diabetic nephropathy
Abstract
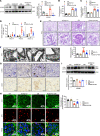
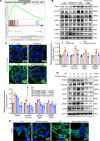
Albuminuria and podocyte injury are the key cellular events in the progression of diabetic nephropathy (DN). Acetyl-CoA synthetase 2 (ACSS2) is a nucleocytosolic enzyme responsible for the regulation of metabolic homeostasis in mammalian cells. This study aimed to investigate the possible roles of ACSS2 in kidney injury in DN. We constructed an ACSS2-deleted mouse model to investigate the role of ACSS2 in podocyte dysfunction and kidney injury in diabetic mouse models. In vitro, podocytes were chosen and transfected with ACSS2 siRNA and ACSS2 inhibitor and treated with high glucose. We found that ACSS2 expression was significantly elevated in the podocytes of patients with DN and diabetic mice. ACSS2 upregulation promoted phenotype transformation and inflammatory cytokine expression while inhibiting podocytes' autophagy. Conversely, ACSS2 inhibition improved autophagy and alleviated podocyte injury. Furthermore, ACSS2 epigenetically activated raptor expression by histone H3K9 acetylation, promoting activation of the mammalian target of rapamycin complex 1 (mTORC1) pathway. Pharmacological inhibition or genetic depletion of ACSS2 in the streptozotocin-induced diabetic mouse model greatly ameliorated kidney injury and podocyte dysfunction. To conclude, ACSS2 activation promoted podocyte injury in DN by raptor/mTORC1-mediated autophagy inhibition.
Keywords: Diabetes; Metabolism; Molecular biology; Nephrology.
Conflict of interest statement
Figures





References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

