Serological response and breakthrough infection after COVID-19 vaccination in patients with cirrhosis and post-liver transplant
- PMID: 37870985
- PMCID: PMC10586829
- DOI: 10.1097/HC9.0000000000000273
Serological response and breakthrough infection after COVID-19 vaccination in patients with cirrhosis and post-liver transplant
Abstract
Background: Vaccine hesitancy and lack of access remain major issues in disseminating COVID-19 vaccination to liver patients globally. Factors predicting poor response to vaccination and risk of breakthrough infection are important data to target booster vaccine programs. The primary aim of the current study was to measure humoral responses to 2 doses of COVID-19 vaccine. Secondary aims included the determination of factors predicting breakthrough infection.
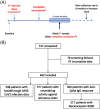
Methods: COVID-19 vaccination and Biomarkers in cirrhosis And post-Liver Transplantation is a prospective, multicenter, observational case-control study. Participants were recruited at 4-10 weeks following first and second vaccine doses in cirrhosis [n = 325; 94% messenger RNA (mRNA) and 6% viral vaccine], autoimmune liver disease (AILD) (n = 120; 77% mRNA and 23% viral vaccine), post-liver transplant (LT) (n = 146; 96% mRNA and 3% viral vaccine), and healthy controls (n = 51; 72% mRNA, 24% viral and 4% heterologous combination). Serological end points were measured, and data regarding breakthrough SARS-CoV-2 infection were collected.
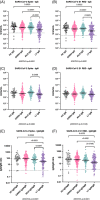
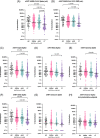
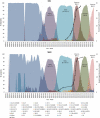
Results: After adjusting by age, sex, and time of sample collection, anti-Spike IgG levels were the lowest in post-LT patients compared to cirrhosis (p < 0.0001), AILD (p < 0.0001), and control (p = 0.002). Factors predicting reduced responses included older age, Child-Turcotte-Pugh B/C, and elevated IL-6 in cirrhosis; non-mRNA vaccine in AILD; and coronary artery disease, use of mycophenolate and dysregulated B-call activating factor, and lymphotoxin-α levels in LT. Incident infection occurred in 6.6%, 10.6%, 7.4%, and 15.6% of cirrhosis, AILD, post-LT, and control, respectively. The only independent factor predicting infection in cirrhosis was low albumin level.
Conclusions: LT patients present the lowest response to the SARS-CoV-2 vaccine. In cirrhosis, the reduced response is associated with older age, stage of liver disease and systemic inflammation, and breakthrough infection with low albumin level.
Copyright © 2023 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the American Association for the Study of Liver Diseases.
Conflict of interest statement
Gautam Mehta owns stock in Hepyx Ltd. Mar Riveiro-Barciela advises GSK and received grants from Gilead. Angel Sangiovanni is on the speakers’ bureau for Abbott. Xavier Verhelst consults for and received grants from AbbVie. He consults for Eisai. He received grants from Gilead, Dr. Falk Pharma, and Astellas. Vishal C Patel received grants from Oxford Nanopore Technologies. Amir Gander owns stock in Tissue Access for Patient Benefit Limited. Maria Francesca Donato advises and is on the speakers’ bureau for Kedrion Biopharma. Pierluigi Toniutto consults for, is on the speakers’ bureau for, and received grants from Gilead. He consults for and is on the speakers’ bureau for MSD. He is on the speakers’ bureau for and received grants from Intercept. He is on the speakers’ bureau for MSD, AbbVie, Shionogi, SOBI, and Novartis. Thomas Berg advises, is on the speakers’ bureau of, and received grants from Ipsen. He advises and is on the speakers’ bureau for Eisai, Roche, and Bayer. Francesco Paolo Russo is on the speakers’ bureau for AbbVie and Gilead. Marina Berenguer consults for Gilead, Ipsen, Orphalan, Deep-genomic, Alexion, and Chiesi. She received grants from Gilead. Vincente Arroyo has other interests in Grifols. Paolo Angeli is on the speakers’ bureau for and received grants from CSL Behring. He advises BioVie, BioMarin, and Genfit. He is on the speakers’ bureau for Grifols and Kedrion. Salvatore Piano consults for Plasma Protein Therapeutics Association and Resolution Therapeutics. He advises Mallinckrodt. He is on the speakers’ bureau for Grifols. Rajiv Jalan owns stock in Yaqrit, Hepyx, Cyberliver, and Gigabiome. The remaining authors have nothing to report.
Figures
Comment in
-
Reply to: Serological response after COVID-19 vaccination, cirrhosis, and postliver transplant.Hepatol Commun. 2024 May 10;8(6):e0421. doi: 10.1097/HC9.0000000000000421. eCollection 2024 Jun 1. Hepatol Commun. 2024. PMID: 38780311 Free PMC article. No abstract available.
References
-
- Accessed 9, January 2023. https://ourworldindata.org/covid-vaccinations
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

