Single-Cell Transcriptome Profiling of Pancreatic Islets From Early Diabetic Mice Identifies Anxa10 for Ca2+ Allostasis Toward β-Cell Failure
- PMID: 37871012
- PMCID: PMC10784657
- DOI: 10.2337/db23-0212
Single-Cell Transcriptome Profiling of Pancreatic Islets From Early Diabetic Mice Identifies Anxa10 for Ca2+ Allostasis Toward β-Cell Failure
Abstract
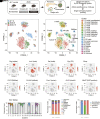
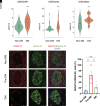
Type 2 diabetes is a progressive disorder denoted by hyperglycemia and impaired insulin secretion. Although a decrease in β-cell function and mass is a well-known trigger for diabetes, the comprehensive mechanism is still unidentified. Here, we performed single-cell RNA sequencing of pancreatic islets from prediabetic and diabetic db/db mice, an animal model of type 2 diabetes. We discovered a diabetes-specific transcriptome landscape of endocrine and nonendocrine cell types with subpopulations of β- and α-cells. We recognized a new prediabetic gene, Anxa10, that was induced by and regulated Ca2+ influx from metabolic stresses. Anxa10-overexpressed β-cells displayed suppression of glucose-stimulated intracellular Ca2+ elevation and potassium-induced insulin secretion. Pseudotime analysis of β-cells predicted that this Ca2+-surge responder cluster would proceed to mitochondria dysfunction and endoplasmic reticulum stress. Other trajectories comprised dedifferentiation and transdifferentiation, emphasizing acinar-like cells in diabetic islets. Altogether, our data provide a new insight into Ca2+ allostasis and β-cell failure processes.
Article highlights: The transcriptome of single-islet cells from healthy, prediabetic, and diabetic mice was studied. Distinct β-cell heterogeneity and islet cell-cell network in prediabetes and diabetes were found. A new prediabetic β-cell marker, Anxa10, regulates intracellular Ca2+ and insulin secretion. Diabetes triggers β-cell to acinar cell transdifferentiation.
© 2023 by the American Diabetes Association.
Conflict of interest statement
Figures






References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

