Escherichia coli utilizes multiple peptidoglycan recycling permeases with distinct strategies of recycling
- PMID: 37871219
- PMCID: PMC10622912
- DOI: 10.1073/pnas.2308940120
Escherichia coli utilizes multiple peptidoglycan recycling permeases with distinct strategies of recycling
Abstract
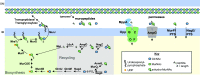
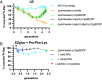
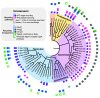
Bacteria produce a structural layer of peptidoglycan (PG) that enforces cell shape, resists turgor pressure, and protects the cell. As bacteria grow and divide, the existing layer of PG is remodeled and PG fragments are released. Enterics such as Escherichia coli go to great lengths to internalize and reutilize PG fragments. E. coli is estimated to break down one-third of its cell wall, yet only loses ~0 to 5% of meso-diaminopimelic acid, a PG-specific amino acid, per generation. Two transporters were identified early on to possibly be the primary permease that facilitates PG fragment recycling, i) AmpG and ii) the Opp ATP binding cassette transporter in conjunction with a PG-specific periplasmic binding protein, MppA. The contribution of each transporter to PG recycling has been debated. Here, we have found that AmpG and MppA/Opp are differentially regulated by carbon source and growth phase. In addition, MppA/Opp is uniquely capable of high-affinity scavenging of muropeptides from growth media, demonstrating that AmpG and MppA/Opp allow for different strategies of recycling PG fragments. Altogether, this work clarifies environmental contexts under which E. coli utilizes distinct permeases for PG recycling and explores how scavenging by MppA/Opp could be beneficial in mixed communities.
Keywords: AmpG; cell wall; muropeptides; peptidoglycan; peptidoglycan recycling.
Conflict of interest statement
The authors declare no competing interest.
Figures



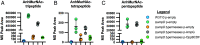

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

