Infusion Product TNFα, Th2, and STAT3 Activities Are Associated with Clinical Responses to Transgenic T-cell Receptor Cell Therapy
- PMID: 37871333
- PMCID: PMC10702871
- DOI: 10.1158/2326-6066.CIR-23-0577
Infusion Product TNFα, Th2, and STAT3 Activities Are Associated with Clinical Responses to Transgenic T-cell Receptor Cell Therapy
Abstract
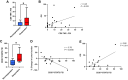
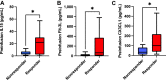
Transgenic T-cell receptor (TCR) T cell-based adoptive cell therapies for solid tumors are associated with dramatic initial response rates, but there remain many instances of treatment failure and disease relapse. The association of infusion product cytokine profiles with clinical response has not been explored in the context of TCR T-cell therapy products. Single-cell antigen-dependent secretomic and proteomic analysis of preinfusion clinical TCR T-cell therapy products revealed that TNFα cytokine functionality of CD8+ T cells and phospho-STAT3 signaling in these cells were both associated with superior clinical responsiveness to therapy. By contrast, CD4+ T-helper 2 cell cytokine profiles were associated with inferior clinical responses. In parallel, preinfusion levels of IL15, Flt3-L, and CX3CL1 were all found to be associated with clinical response to therapy. These results have implications for the development of therapeutic biomarkers and identify potential targets for enrichment in the design of transgenic TCR T-cell therapies for solid tumors.
©2023 The Authors; Published by the American Association for Cancer Research.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

