Cardiomyocyte NOX4 regulates resident macrophage-mediated inflammation and diastolic dysfunction in stress cardiomyopathy
- PMID: 37871532
- PMCID: PMC10598408
- DOI: 10.1016/j.redox.2023.102937
Cardiomyocyte NOX4 regulates resident macrophage-mediated inflammation and diastolic dysfunction in stress cardiomyopathy
Abstract
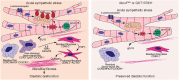
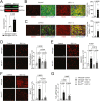
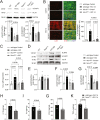
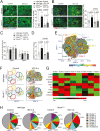
In acute sympathetic stress, catecholamine overload can lead to stress cardiomyopathy. We tested the hypothesis that cardiomyocyte NOX4 (NADPH oxidase 4)-dependent mitochondrial oxidative stress mediates inflammation and diastolic dysfunction in stress cardiomyopathy. Isoproterenol (ISO; 5 mg/kg) injection induced sympathetic stress in wild-type and cardiomyocyte (CM)-specific Nox4 knockout (Nox4CM-/-) mice. Wild-type mice treated with ISO showed higher CM NOX4 expression, H2O2 levels, inflammasome activation, and IL18, IL6, CCL2, and TNFα levels than Nox4CM-/- mice. Spectral flow cytometry and t-SNE analysis of cardiac cell suspensions showed significant increases in pro-inflammatory and pro-fibrotic embryonic-derived resident (CCR2-MHCIIhiCX3CR1hi) macrophages in wild-type mice 3 days after ISO treatment, whereas Nox4CM-/- mice had a higher proportion of embryonic-derived resident tissue-repair (CCR2-MHCIIloCX3CR1lo) macrophages. A significant increase in cardiac fibroblast activation and interstitial collagen deposition and a restrictive pattern of diastolic dysfunction with increased filling pressure was observed in wild-type hearts compared with Nox4CM-/- 7 days post-ISO. A selective NOX4 inhibitor, GKT137831, reduced myocardial mitochondrial ROS, macrophage infiltration, and fibrosis in ISO-injected wild-type mice, and preserved diastolic function. Our data suggest sympathetic overstimulation induces resident macrophage (CCR2-MHCII+) activation and myocardial inflammation, resulting in fibrosis and impaired diastolic function mediated by CM NOX4-dependent ROS.
Keywords: Cardiac fibroblasts; Cardiomyocyte mitochondria; Cardiomyopathy; Diastolic dysfunction; NADPH oxidase 4; Resident macrophages.
Copyright © 2023 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of competing interest Marschall S. Runge is a member of the Board of Directors at Eli Lilly and Company. Other authors have declared that no conflict of interest exists.
Figures


References
-
- Templin C., Ghadri J.R., Diekmann J., Napp L.C., Bataiosu D.R., Jaguszewski M., Cammann V.L., Sarcon A., Geyer V., Neumann C.A., Seifert B., Hellermann J., Schwyzer M., Eisenhardt K., Jenewein J., Franke J., Katus H.A., Burgdorf C., Schunkert H., Moeller C., Thiele H., Bauersachs J., Tschope C., Schultheiss H.P., Laney C.A., Rajan L., Michels G., Pfister R., Ukena C., Bohm M., Erbel R., Cuneo A., Kuck K.H., Jacobshagen C., Hasenfuss G., Karakas M., Koenig W., Rottbauer W., Said S.M., Braun-Dullaeus R.C., Cuculi F., Banning A., Fischer T.A., Vasankari T., Airaksinen K.E., Fijalkowski M., Rynkiewicz A., Pawlak M., Opolski G., Dworakowski R., MacCarthy P., Kaiser C., Osswald S., Galiuto L., Crea F., Dichtl W., Franz W.M., Empen K., Felix S.B., Delmas C., Lairez O., Erne P., Bax J.J., Ford I., Ruschitzka F., Prasad A., Luscher T.F. Clinical features and outcomes of Takotsubo (stress) cardiomyopathy. N. Engl. J. Med. 2015;373:929–938. doi: 10.1056/NEJMoa1406761. - DOI - PubMed
-
- Neil C., Nguyen T.H., Kucia A., Crouch B., Sverdlov A., Chirkov Y., Mahadavan G., Selvanayagam J., Dawson D., Beltrame J., Zeitz C., Unger S., Redpath T., Frenneaux M., Horowitz J. Slowly resolving global myocardial inflammation/oedema in Tako-Tsubo cardiomyopathy: evidence from T2-weighted cardiac MRI. Heart. 2012;98:1278–1284. doi: 10.1136/heartjnl-2011-301481. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

