AMPKα1 negatively regulates osteoclastogenesis and mitigates pathological bone loss
- PMID: 37871745
- PMCID: PMC10692901
- DOI: 10.1016/j.jbc.2023.105379
AMPKα1 negatively regulates osteoclastogenesis and mitigates pathological bone loss
Abstract
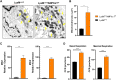
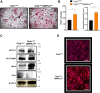
Osteoclasts are specialized cells responsible for bone resorption, a highly energy-demanding process. Focus on osteoclast metabolism could be a key for the treatment of osteolytic diseases including osteoporosis. In this context, AMP-activated protein kinase α1 (AMPKα1), an energy sensor highly expressed in osteoclasts, participates in the metabolic reconfiguration during osteoclast differentiation and activation. This study aimed to elucidate the role of AMPKα1 during osteoclastogenesis in vitro and its impact in bone loss in vivo. Using LysMcre/0AMPK⍺1f/f animals and LysMcre/0 as control, we evaluated how AMPKα1 interferes with osteoclastogenesis and bone resorption activity in vitro. We found that AMPKα1 is highly expressed in the early stages of osteoclastogenesis. Genetic deletion of AMPKα1 leads to increased gene expression of osteoclast differentiation and fusion markers. In addition, LysMcre/0AMPK⍺1f/f mice had an increased number and size of differentiated osteoclast. Accordingly, AMPKα1 negatively regulates bone resorption in vitro, as evidenced by the area of bone resorption in LysMcre/0AMPK⍺1f/f osteoclasts. Our data further demonstrated that AMPKα1 regulates mitochondrial fusion and fission markers upregulating Mfn2 and downregulating DRP1 (dynamics-related protein 1) and that Ctskcre/0AMPK⍺1f/f osteoclasts lead to an increase in the number of mitochondria in AMPK⍺1-deficient osteoclast. In our in vivo study, femurs from Ctskcre/0AMPK⍺1f/f animals exhibited bone loss associated with the increased number of osteoclasts, and there was no difference between Sham and ovariectomized group. Our data suggest that AMPKα1 acts as a negative regulator of osteoclastogenesis, and the depletion of AMPKα1 in osteoclast leads to a bone loss state similar to that observed after ovariectomy.
Keywords: AMPK; metabolism; osteoclast; osteoclastogenesis; osteoporosis.
Copyright © 2023 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest The authors declare that they have no conflicts of interest with the contents of this article.
Figures


References
-
- Armstrong A.P., Tometsko M.E., Glaccum M., Sutherland C.L., Cosman D., Dougall W.C. A RANK/TRAF6-dependent signal transduction pathway is essential for osteoclast cytoskeletal organization and resorptive function. J. Biol. Chem. 2002;277:44347–44356. - PubMed
-
- Lemma S., Sboarina M., Porporato P.E., Zini N., Sonveaux P., Di Pompo G., et al. Energy metabolism in osteoclast formation and activity. Int. J. Biochem. Cell Biol. 2016;79:168–180. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

