Angular Head Velocity Cells within Brainstem Nuclei Projecting to the Head Direction Circuit
- PMID: 37871964
- PMCID: PMC10711713
- DOI: 10.1523/JNEUROSCI.0581-23.2023
Angular Head Velocity Cells within Brainstem Nuclei Projecting to the Head Direction Circuit
Abstract
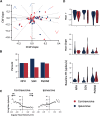
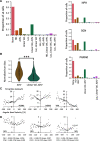
The sense of orientation of an animal is derived from the head direction (HD) system found in several limbic structures and depends on an intact vestibular labyrinth. However, how the vestibular system influences the generation and updating of the HD signal remains poorly understood. Anatomical and lesion studies point toward three key brainstem nuclei as key components for generating the HD signal-nucleus prepositus hypoglossi, supragenual nucleus, and dorsal paragigantocellularis reticular nuclei. Collectively, these nuclei are situated between the vestibular nuclei and the dorsal tegmental and lateral mammillary nuclei, which are thought to serve as the origin of the HD signal. To determine the types of information these brain areas convey to the HD network, we recorded neurons from these regions while female rats actively foraged in a cylindrical enclosure or were restrained and rotated passively. During foraging, a large subset of cells in all three nuclei exhibited activity that correlated with the angular head velocity (AHV) of the rat. Two fundamental types of AHV cells were observed; (1) symmetrical AHV cells increased or decreased their firing with increases in AHV regardless of the direction of rotation, and (2) asymmetrical AHV cells responded differentially to clockwise and counterclockwise head rotations. When rats were passively rotated, some AHV cells remained sensitive to AHV, whereas firing was attenuated in other cells. In addition, a large number of AHV cells were modulated by linear head velocity. These results indicate the types of information conveyed from the vestibular nuclei that are responsible for generating the HD signal.SIGNIFICANCE STATEMENT Extracellular recording of brainstem nuclei (nucleus prepositus hypoglossi, supragenual nucleus, and dorsal paragigantocellularis reticular nucleus) that project to the head direction circuit identified different types of AHV cells while rats freely foraged in a cylindrical environment. The firing of many cells was also modulated by linear velocity. When rats were restrained and passively rotated, some cells remained sensitive to AHV, whereas others had attenuated firing. These brainstem nuclei provide critical information about the rotational movement of the head of the rat in the azimuthal plane.
Keywords: angular head velocity; head direction; navigation; nucleus prepositus; spatial orientation; supragenual nucleus.
Copyright © 2023 the authors.
Figures









Update of
-
Angular head velocity cells within brainstem nuclei projecting to the head direction circuit.bioRxiv [Preprint]. 2023 Mar 31:2023.03.29.534808. doi: 10.1101/2023.03.29.534808. bioRxiv. 2023. Update in: J Neurosci. 2023 Dec 6;43(49):8403-8424. doi: 10.1523/JNEUROSCI.0581-23.2023. PMID: 37034640 Free PMC article. Updated. Preprint.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
