Participant Experience with Protocol Research Kidney Biopsies in the Kidney Precision Medicine Project
- PMID: 37871973
- PMCID: PMC10861112
- DOI: 10.2215/CJN.0000000000000334
Participant Experience with Protocol Research Kidney Biopsies in the Kidney Precision Medicine Project
Abstract
Background: Kidney biopsies are procedures commonly performed in clinical nephrology and are increasingly used in research. In this study, we aimed to evaluate the experiences of participants who underwent research kidney biopsies in the Kidney Precision Medicine Project (KPMP).
Methods: KPMP research participants with AKI or CKD were enrolled at nine recruitment sites in the United States between September 2019 and January 2023. At 28 days postbiopsy, participants were invited to complete a survey to share their experiences, including motivation to participate in research, comprehension of informed consent, pain and anxiety during and after the biopsy procedure, overall satisfaction with KPMP participation, and effect of the study on their lives. The survey was developed in collaboration with the KPMP Community Engagement Committee and the Institute of Translational Health Sciences at the University of Washington.
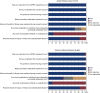
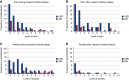
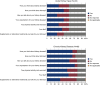
Results: One hundred and eleven participants completed the survey, 23 enrolled for AKI and 88 for CKD. The median age was 61 (interquartile range [IQR], 48-67) years, 43% were women, 28% were Black, and 18% were of Hispanic ethnicity. Survey respondents most commonly joined KPMP to help future patients (59%). The consent form was understood by 99%, and 97% recognized their important role in this study. Pain during the biopsy was reported by 50%, at a median level of 1 (IQR, 0-3) on a 0-10 scale. Anxiety during the biopsy was described by 64% at a median level of 3 (IQR, 1-5) on a 0-10 scale. More than half conveyed that KPMP participation had an effect on their diet, physical activity, and how they think about kidney disease.
Conclusions: KPMP survey respondents were most commonly motivated to participate in research protocol kidney biopsies by altruism, with excellent understanding of the informed consent process.
Podcast: This article contains a podcast at https://dts.podtrac.com/redirect.mp3/www.asn-online.org/media/podcast/CJASN/2023_11_20_CJN0000000000000334.mp3This article contains a podcast at https://dts.podtrac.com/redirect.mp3/www.asn-online.org/media/podcast/CJASN/2023_11_20_Spanish_CJN00000000.mp3.
Copyright © 2023 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the American Society of Nephrology.
Conflict of interest statement
S.W. Chen reports employment with Brigham and Women's Hospital and Joslin Diabetes Center. C.P. Corona-Villalobos reports employment with Johns Hopkins University. I.H. de Boer reports consultancy for Alnylam, AstraZeneca, Bayer, Boehringer-Ingelheim, Boehringer-Ingelheim/Lilly, George Clinical, Gilead, Medscape, and Otsuka; research funding from DexCom and Novo Nordisk; honoraria from National Intitutes of Health; and advisory or leadership roles as a Deputy Editor of
Figures


References
-
- Planner C, Bower P, Donnelly A, Gillies K, Turner K, Young B. Trials need participants but not their feedback? A scoping review of published papers on the measurement of participant experience of taking part in clinical trials. Trials. 2019;20(1):381. doi: 10.1186/s13063-019-3444-y - DOI - PMC - PubMed
-
- Institute Medicine. The CTSA Program at NIH: Opportunities for Advancing Clinical and Translational Research. National Academies Press; 2013;18323. Accessed April 24, 2022. http://www.nap.edu/catalog/18323 doi: 10.17226/18323 - DOI - PubMed
MeSH terms
Grants and funding
- R01 DK121019/DK/NIDDK NIH HHS/United States
- U01 DK114866/DK/NIDDK NIH HHS/United States
- U01 DK133090/DK/NIDDK NIH HHS/United States
- U01 DK114933/DK/NIDDK NIH HHS/United States
- U01 DK114908/DK/NIDDK NIH HHS/United States
- U01 DK133095/DK/NIDDK NIH HHS/United States
- U01 DK133081/DK/NIDDK NIH HHS/United States
- U01 DK114907/DK/NIDDK NIH HHS/United States
- U01 DK114920/DK/NIDDK NIH HHS/United States
- U24 DK114886/DK/NIDDK NIH HHS/United States
- U01 DK133766/DK/NIDDK NIH HHS/United States
- U01 DK114923/DK/NIDDK NIH HHS/United States
- U01 DK133113/DK/NIDDK NIH HHS/United States
- U01 DK133097/DK/NIDDK NIH HHS/United States
- U01DK133081, U01DK133091, U01DK133092, U01DK133093, U01DK133095, U01DK133097, U01DK114866, U01DK114908, U01DK133090, U01DK133113, U01DK133766, U01DK133768, U01DK114907, U01DK114920, U01DK114923, U01DK114933, U24DK114886./DK/NIDDK NIH HHS/United States
- U01 DK133768/DK/NIDDK NIH HHS/United States
- U01 DK133092/DK/NIDDK NIH HHS/United States
- U01 DK133091/DK/NIDDK NIH HHS/United States
- U01 DK133093/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources

