Targeted depletion of TRBV9+ T cells as immunotherapy in a patient with ankylosing spondylitis
- PMID: 37872223
- PMCID: PMC10667094
- DOI: 10.1038/s41591-023-02613-z
Targeted depletion of TRBV9+ T cells as immunotherapy in a patient with ankylosing spondylitis
Abstract
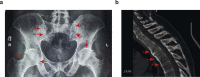
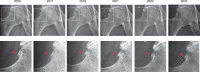
Autoimmunity is intrinsically driven by memory T and B cell clones inappropriately targeted at self-antigens. Selective depletion or suppression of self-reactive T cells remains a holy grail of autoimmune therapy, but disease-associated T cell receptors (TCRs) and cognate antigenic epitopes remained elusive. A TRBV9-containing CD8+ TCR motif was recently associated with the pathogenesis of ankylosing spondylitis, psoriatic arthritis and acute anterior uveitis, and cognate HLA-B*27-presented epitopes were identified. Following successful testing in nonhuman primate models, here we report human TRBV9+ T cell elimination in ankylosing spondylitis. The patient achieved remission within 3 months and ceased anti-TNF therapy after 5 years of continuous use. Complete remission has now persisted for 4 years, with three doses of anti-TRBV9 administered per year. We also observed a profound improvement in spinal mobility metrics and the Bath Ankylosing Spondylitis Metrology Index (BASMI). This represents a possibly curative therapy of an autoimmune disease via selective depletion of a TRBV-defined group of T cells. The anti-TRBV9 therapy could potentially be applicable to other HLA-B*27-associated spondyloarthropathies. Such targeted elimination of the underlying cause of the disease without systemic immunosuppression could offer a new generation of safe and efficient therapies for autoimmunity.
© 2023. The Author(s).
Conflict of interest statement
A.A.A., Y.Y.U., A.K.V. and P.A.I. are employees of BIOCAD. A.N.D. and M.I. are employees of MiLaboratories. E.S.E. is an employee of Miltenyi Biotec. The other authors declare no competing interests.
Figures





References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

